Gonorrhea is the second most common notifiable sexually transmitted infection (STI) in the United States. This infection is caused by the gram-negative bacterium Neisseria gonorrhoeae and is transmitted through sexual activity or perinatally during vaginal delivery. Reported cases of gonorrhea have remained high in recent years, particularly among sexually active persons who are 20 to 29 years of age. The most common clinical sites for gonococcal infection are the urethra, cervix, pharynx, or rectum. In the modern era, there are excellent diagnostic tests for gonorrhea. Due to antimicrobial resistance concerns, the treatment recommendations for gonorrhea have recently changed. Aggressive screening and public health measures are needed to reduce N. gonorrhoeae transmission rates.
- Module 2 Overview
Self-Study Lessons - 0%Lesson 1
Chlamydial InfectionsActivities- 0%Lesson 2
Gonococcal InfectionsActivities- 0%Lesson 3
SyphilisActivities- 0%Lesson 5
Genital HerpesActivities- 0%Lesson 6
Human Papillomavirus InfectionActivities- 0%Lesson 7
Pelvic Inflammatory DiseaseActivities- 0%Lesson 8
VaginitisActivities- 0%Lesson 9
MpoxActivities- 0%Lesson 10
Mycoplasma genitaliumActivitiesLesson 2. Gonococcal Infections
PDF ShareLast Updated: March 20th, 2025Authors:David H. Spach, MD,David H. Spach, MD
Editor-in-Chief
Professor of Medicine
Division of Allergy and Infectious Diseases
University of WashingtonDisclosures: NoneHilary Reno, MD, PhD,Hilary Reno, MD, PhD
Professor of Medicine
Principal Investigator, St. Louis STI/PTC
Washington University in St. LouisDisclosures: Grant to Institution: Hologic, Inc.
Presentation Development: Prime Inc.Jehan Z. Budak, MDJehan Z. Budak, MD
Assistant Professor of Medicine
Division of Allergy & Infectious Diseases
University of WashingtonDisclosures: NoneLearning Objective Performance Indicators
- Recognize the clinical manifestations associated with gonococcal infections
- Use optimal laboratory diagnostic methods to diagnose gonococcal infections
- Perform routine screening for gonococcal infection when indicated
- Administer recommended treatment regimens for gonococcal infections
- Manage sex partners of persons diagnosed with gonococcal infection
Table of Contents- Gonococcal Infections
- Introduction
- Epidemiology in the United States
- Gonococcal Antimicrobial Susceptibility
- Microbiology, Pathogenesis, and Transmission
- Clinical Manifestations
- Laboratory Diagnosis
- Screening for Gonococcal Infection
- Women
- Women Who Have Sex with Women (WSW) and Women Who Have Sex with Women and Men
- Pregnant Women
- Men Who Have Sex Only with Women
- Men Who Have Sex with Men
- Persons with HIV
- Persons Receiving HIV Preexposure Prophylaxis (HIV PrEP)
- Persons Receiving Postexposure Prophylaxis (Doxy PEP)
- Persons in Correctional Facilities
- Treatment
- Treatment of Gonococcal Infections
- Uncomplicated Infections of the Cervix, Urethra, and Rectum
- Uncomplicated Infections of the Pharynx
- Conjunctivitis
- Disseminated Gonococcal Infection
- Allergy to Penicillin or Cephalosporin
- Gonococcal Infections in Pregnancy
- Management of Antibiotic-Resistant Gonorrhea
- Defining Gonococcal Treatment Failure
- Management of Suspected Gonococcal Treatment Failure
- Follow-Up
- Management of Sex Partners
- Counseling and Education
- Prevention
- Summary Points
- Check-On-Learning
- Citations
- Additional References
- Figures
- Tables
Introduction
Epidemiology in the United States
2023 Gonorrhea Surveillance Data
In 2023, a total of 601,319 cases of Neisseria gonorrhoeae (gonorrhea) were reported in the United States, making gonorrhea the second-highest reported bacterial STI (Figure 1).[1] Since reporting of gonorrhea began in the United States in the early 1940s, there have been several major trends—a steady rise in cases during the 1950s and 1960s, a peak in cases during the mid-1970s, a subsequent decline until a historic low in 2009, a significant increase from 2010 through 2021 and, most recently, a decrease in 2022 and 2023.[1] There are significant differences in the incidence of gonorrhea based on sex, age, race/ethnicity, and region of residence.[1] The following summarizes several key epidemiologic features of gonococcal infections as reported in the United States for the year 2023.[1]
- Sex: Since 2014, the rates of reported gonorrhea cases among males have been significantly higher than in females. In 2023, the gonorrhea rate (cases per 100,000 population) was 228.3 in males versus 130.7 in females.
- Age: Based on age group, the highest rate of gonorrhea cases for both males and females occurred among persons 20 to 24 years of age. The age group with the second-highest rate for males was individuals 25–29 years of age, and for females, it was individuals 15–19 years of age.
- Race/Ethnicity: The gonorrhea rates (cases per 100,000 persons) among different racial/ethnic groups in the United States were highest among Black persons, with the next highest rates in American Indian/Alaska Native persons. The total number of gonorrhea cases was highest in Black people and next highest in White people.
- Geographic Region: The overall rate of gonorrhea cases in the United States was 179.5 per 100,000 people. The highest reported rates of gonorrhea were in the South. The five states with the highest rates in 2023 (in descending order) were Alaska, Louisiana, Georgia, South Dakota, and Mississippi; the gonorrhea rate in the District of Columbia was 853.3 per 100,000 population, which was higher than in any state.
Impact
Gonococcal infections are a significant public health problem in the United States. Based on the estimated incident cases among all ages in 2018, the total lifetime direct medical cost of gonorrhea in the United States was estimated at $271 million.[2]Gonococcal Antimicrobial Susceptibility
Antimicrobial resistance is an important consideration in the treatment of gonorrhea.[3,4,5] Much of the information regarding antimicrobial susceptibility of N. gonorrhoeae isolates in the United States has come from the Centers for Disease Control and Prevention (CDC) Gonococcal Isolate Surveillance Project (GISP).[6] The higher the minimum inhibitory concentrations (MICs) in clinical isolates, the greater the antimicrobial concentration needed to inhibit the growth of N. gonorrhoeae. Increases above a defined cutoff indicate an elevated risk of treatment failure or impending resistance, often referred to as alert values. The GISP tracks trends in antimicrobial susceptibility in the United States to seven antimicrobials that are currently or previously used for gonorrhea treatment: azithromycin, cefixime, ceftriaxone, ciprofloxacin, gentamicin, penicillin, and tetracycline.[6] The following data summarizes data from the 2022 GISP (Figure 2).[6]
- Azithromycin: From 1998-2012, the percentage of isolates with reduced azithromycin susceptibility (MIC ≥2.0 μg/mL) was consistently less than 1%, but the resistance rates have consistently increased since then. During the 2018-2022 time period, 5.2% of the gonococcal isolates had an MIC ≥2.0 μg/mL.
- Cefixime: Since 2013, the percentage of gonococcal isolates with cefixime MICs ≥0.25 μg/mL has consistently been less than 1%. In 2022, there were 0.1% of gonococcal isolates with cefixime MICs ≥0.25 μg/mL.
- Ceftriaxone: Since 1998, the percentage of isolates with reduced ceftriaxone susceptibility (MIC ≥0.125 μg/mL) has remained less than 0.5%. During 2018-2022, the percentage of isolates with reduced susceptibility to ceftriaxone (MICs ≥0.125 μg/mL) was 0.1%. In November 2019, the Southern Nevada Public Health Laboratory identified a clinical N. gonorrhoeae isolate with a confirmed ceftriaxone MIC of 1.0 µg/mL, which is the highest ceftriaxone MIC identified in GISP to date.[7]
- Ciprofloxacin: Fluoroquinolone-resistant N. gonorrhoeae is widely disseminated throughout the United States (and globally), and rates have steadily increased during the past decade. Since 2017, more than 30% of the GISP isolates showed resistance to ciprofloxacin.
- Gentamicin: From 2018-2022, more than 75% of the gonococcal isolates had a gentamicin MIC value of 8 μg/mL. In 2022, among all gonococcal isolates tested, 3.8% had an MIC of ≥16 μg/mL, and 0.2% had an MIC of ≥32 μg/mL. The criteria for gonococcal susceptibility to gentamicin have not been established. Some experts have suggested gonococcal isolates with a gentamicin MIC of less than or equal to 4 μg/L are susceptible, those with an MIC of 8 to 16 μg/L have intermediate susceptibility, and those with an MIC greater than 32 μg/L or greater are resistant.[8,9]
- Penicillin: In 2022, resistance to penicillin (MIC ≥2.0 μg/mL) was detected in 10.7% of gonococcal isolates. Since 2006, the penicillin gonococcal resistance rates have consistently been greater than 10%.
- Tetracycline: In 2022, resistance to tetracycline (MIC ≥2.0 μg/mL) was detected in 20.2% of the gonococcal isolates. Since 2005, the tetracycline gonococcal resistance rates have consistently been greater than 15%.
Microbiology, Pathogenesis, and Transmission
Organism
Gonorrhea is a common bacterial sexually transmitted disease caused by N. gonorrhoeae, a gram-negative bacterium that divides by binary fission and thus usually appears as pairs (diplococci) with hair-like pilli extending from the surface outer membrane (Figure 3). Optimal growth of N. gonorrhoeae requires nutritional supplementation, such as with a Thayer-Martin media. Neisseria gonorrhoeae attaches to different types of epithelial cells via its surface pili, rendering it capable of infecting mucosal surfaces, such as the urogenital epithelium, oropharyngeal tract, and conjunctival tissue.[10,11] This organism has a number of virulence factors, including type IV pili, lipooligosaccharide antigens, outer membrane porin protein (Por), opacity protein (Opa), and IgA protease, that collectively facilitate evasion of the host immune response.[10,11,12] Infection with N. gonorrhoeae generates limited immunity, and thus, repeated infections can occur.[12]
Transmission
Multiple factors have been identified that are associated with an increased risk for acquiring gonorrhea, including multiple or new sex partners, lack of consistent use of condoms, living in an urban area where gonorrhea prevalence is high, age younger than 30 years, and exchange of sex for drugs or money. The transmission of N. gonorrhoeae can occur in several ways:
- Transmission of N. gonorrhoeae from the urethra (in a person with gonorrhea) to the vagina (in a person without gonorrhea) occurs at a rate of approximately 50 to 70% per episode of vaginal intercourse that includes ejaculation; transmission of N. gonorrhoeae can occur from the urethra to the vagina without ejaculation, but at a lower rate.[13]
- Transmission of N. gonorrhoeae from the vagina (in a person with gonorrhea) to the urethra (in a person without gonorrhea) can occur during insertive vaginal intercourse, with an estimated rate of transmission of approximately 20% per episode of intercourse; the transmission rate increases to approximately 60 to 80% after four or more episodes of insertive vaginal intercourse.[14]
- Transmission of N. gonorrhoeae from the genital tract (of a person with gonorrhea) to the pharynx (of a person without gonorrhea) can occur via oral-genital contact; this is best documented with oral-penile contact (fellatio).[15,16] In addition, epidemiologic data have associated acquisition of pharyngeal gonorrhea with kissing and oral-anal contact.[17,18,19]
- Transmission of N. gonorrhoeae from the pharynx of a person with gonorrhea to the urethra (or a person without gonorrhea) can occur during fellatio.[20,21,22]
- Perinatal transmission (mother-to-infant) can occur during vaginal delivery when a mother with gonorrhea has not been treated during the perinatal period.
- Insertive and receptive rectal intercourse gonorrhea transmission rates have not been quantified, but rectal intercourse appears to be an efficient mode of transmission.
- Gonorrhea is associated with increased susceptibility to HIV acquisition, particularly in men who have sex with men (MSM) who have rectal gonorrhea.[23] For men with HIV who are not taking suppressive antiretroviral therapy, urethral gonorrhea is associated with an increase in HIV transmission due to increased urethral HIV shedding.[24]
Clinical Manifestations
Neisseria gonorrhoeae infection can potentially cause an array of clinical syndromes, including urogenital, pharyngeal, and rectal infections in males and females, conjunctivitis in adults and neonates, and uncommonly, disseminated gonococcal infection (DGI). Untreated gonorrhea infection in women can cause pelvic inflammatory disease (PID), tubal infertility, ectopic pregnancy, and chronic pelvic pain.Genital Infection in Men
Urethritis
Urethritis is a common manifestation of gonorrhea in men. Most men develop overt, symptomatic urethritis, but some will develop asymptomatic (unrecognized) infection. Transmission can occur within the window of time between acquisition and onset of symptoms.[25] Asymptomatic gonorrhea may act as a reservoir that perpetuates transmission in the community.[26] The typical symptoms of gonococcal urethritis, when present, include a purulent or mucopurulent urethral discharge (Figure 4), often accompanied by dysuria. Less often, the discharge is clear or cloudy. The incubation period ranges from 1–14 days, with most men becoming symptomatic within 2–5 days after exposure.[27]
Anorectal Infections
Anorectal infection most often occurs in men who have sex with men, with the acquisition of rectal N. gonorrhoeae occurring through receptive anal intercourse. Most men with anorectal gonococcal infection are asymptomatic, although proctitis can occur. Symptoms of proctitis include anal irritation, painful defecation, constipation, scant rectal bleeding, painless mucopurulent discharge, anal pruritus, and tenesmus.[28] When proctitis is suspected, an anoscopic examination is recommended to assess for inflammation and mucosal injury. The range of findings on anoscopy may include normal-appearing anorectal mucosa, purulent discharge, erythema, and easily induced anorectal bleeding.
Complications of Genital Infection in Men
Men with untreated gonococcal genital infection can develop epididymitis, with typical symptoms of unilateral testicular pain and swelling, and epididymal tenderness. Epididymitis is infrequent following gonococcal infection, but it is the most common local complication of gonorrhea infection in men. When it does occur, epididymitis is often associated with overt or subclinical urethritis. Urethral discharge may or may not be present. Other less common complications associated with gonococcal infection in men include inguinal lymphadenitis, penile edema, periurethral abscess or fistula, accessory gland infection (Tyson's glands), balanitis, urethral stricture, prostatitis, and rarely perirectal abscess.
Genital Infection in Women
Cervicitis
Most women with gonococcal infection of the cervix are asymptomatic, but when symptoms occur, they may include nonspecific vaginal discharge, intermenstrual bleeding, dysuria, lower abdominal pain, and dyspareunia.[11,29] Clinically, examination of the cervix may show mucopurulent or purulent cervical discharge (Figure 5) and bleeding of the cervix with minimal contact.[29] The incubation period in women is variable, but symptoms, when they do occur, usually develop within 10 days of the exposure.[30] Approximately 80% of women with genital gonococcal infection have laboratory evidence of urethral infection (urethritis); dysuria may be present, but these women frequently do not have specific urethral symptoms.
Anorectal Infections
Anorectal gonococcal infection is uncommon in women, but it can occur via anal intercourse.[31] In addition, anorectal infection has been reported in women with gonococcal cervicitis who do not acknowledge anal intercourse; presumably, in these women, the anorectal infection results from perineal contamination with infected cervical secretions.
Complications of Genital Infection in Women
There are several complications associated with gonorrhea in women:
- Accessory (Bartholin) Gland Infections: Infection with N. gonorrhea may cause or contribute to an occlusion of the female sex accessory glands (Bartholin’s glands or Skene's glands); this obstruction typically manifests as a unilateral fluid-filled cyst on the labia (Bartholin cyst); infection of this cyst may result in the formation of a painful, tender abscess (Bartholin abscess) (Figure 6).
- Pelvic Inflammatory Disease: If cervical gonococcal infection ascends to the endometrium and/or fallopian tubes, PID may develop, typically causing symptoms that include lower abdominal pain, vaginal discharge, dyspareunia, intermenstrual bleeding, and fever.[32] In some women, PID may also be asymptomatic. Presumptive treatment for PID should be considered if one or more of the following criteria are present on pelvic examination: uterine or adnexal tenderness or cervical motion tenderness. The long-term sequelae of untreated PID can include chronic pelvic pain, tubal infertility, and increased risk for ectopic pregnancy.
- Perihepatitis (Fitz-Hugh-Curtis Syndrome): In situations where gonococcal infection ascends from the cervix, infection may produce inflammation of the liver capsule and the adjacent peritoneum. Most women with perihepatitis have associated PID, but perihepatitis can occur independently. Historically, perihepatitis was attributed only to gonococcal infection, but now it is more often associated with chlamydial infection. Gonococcal perihepatitis is characterized by right upper quadrant pain and may be accompanied by abnormal liver function tests.
Additional Syndromes Seen in Men and Women
Pharyngeal Infection
Overall, most persons diagnosed with pharyngeal gonorrhea are asymptomatic or have a very mild sore throat.[33,34,35] In a longitudinal natural history study involving MSM, most of the men reported a sore throat at some time in the first 1 to 2 weeks after acquiring pharyngeal gonorrhea, followed by asymptomatic carriage for an average of approximately 4 months.[17] Symptoms of pharyngeal gonococcal infection may include pharyngitis, tonsillitis, fever, and cervical adenitis; exudative pharyngitis is rare.[15,36]
Ocular Infection
Gonococcal infection of the eye, when it does occur, typically presents as conjunctivitis. Gonococcal conjunctivitis in adults most often results from autoinoculation in persons with genital gonococcal infection. Persons with gonococcal conjunctivitis may initially develop mild, nonpurulent conjunctivitis that, if untreated, typically progresses to marked conjunctival redness, copious purulent discharge, and conjunctival edema (Figure 7).[37] Less often, ulcerative keratitis develops. Untreated gonococcal conjunctivitis can cause complications that may include corneal perforation, endophthalmitis, and blindness.
Disseminated Gonococcal Infection
Disseminated gonococcal infection is an uncommon but potentially life-threatening systemic infection. Rates of disseminated gonococcal infection decreased during the period 1975-2008, but there have been several outbreaks in the United States in recent years.[18,38] It occurs more often in women than in men, particularly during or shortly after menstruation and during pregnancy.[38] The risk of developing disseminated gonococcal infection is also increased in persons with terminal complement deficiency and in people receiving eculizumab, a medication that inhibits complement activation.[39] Disseminated gonococcal infection is often associated with strains that have a propensity to produce bacteremia without associated urogenital symptoms.[40] To this end, disseminated gonococcal infection is frequently not suspected due to the minimal mucosal inflammation and lack of urogenital symptoms.[40] Clinical manifestations of disseminated gonococcal infection may include a combination of arthralgia, tenosynovitis, arthritis, skin lesions, hepatitis, myocarditis, endocarditis, and meningitis (Figure 8).[41,42]
Infection in Children
Perinatal infections most often occur during childbirth when the neonatal conjunctiva, pharynx, respiratory tract, or anal canal may become infected. Conjunctivitis (ophthalmia neonatorum) is preventable by ocular antimicrobial prophylaxis in the newborn. All cases of gonorrhea beyond the newborn period should be considered possible evidence of sexual abuse. Vulvovaginitis (not cervicitis) is the most common manifestation in sexually abused prepubescent girls. Signs and symptoms may include vaginal discharge (often purulent or crusting), dysuria, odor, irritation, and pruritus. The anorectum and the pharynx are the most frequently infected sites in sexually abused boys; urethritis is less frequently seen. If specimens are to be collected, proper guidelines for collecting forensic evidence must be followed. When evaluating a child who has potentially suffered sexual abuse, the clinician should consult individual state laws concerning reporting and counseling.
Laboratory Diagnosis
The approach to diagnostic testing for N. gonorrhoeae has evolved from traditional cultivation to the widespread use of nucleic acid amplification tests (NAATs).[43] Gram stain, another non-culture test, can be used if available for the diagnosis of urethral gonorrhea in symptomatic males. Culture is still recommended if antimicrobial resistance is a concern, especially in cases of suspected treatment failure.
Nucleic Acid Detection Tests
The NAATs used for detecting N. gonorrhoeae in the United States include polymerase chain reaction tests (Roche Amplicor and Cepheid GeneXpert CT/NG), transcription-mediated amplification (Gen-Probe Aptima), and strand displacement amplification (Becton-Dickinson BDProbeTec ET). Amplified tests are Food and Drug Administration (FDA)-cleared for endocervical specimens from women, urethral specimens from men, and urine specimens from men and women.[43,44,45] Some NAATs are also cleared for vaginal swabs. In May 2019, the FDA cleared two NAATs (Aptima Combo 2 Assay and the Xpert CT/NG) for extragenital diagnostic testing of N. gonorrhoeae and Chlamydia trachomatis in rectal and pharyngeal samples.[46] Multiple studies have shown NAATs are the most sensitive test to detect N. gonorrhoeae infections. At present, antimicrobial susceptibility cannot be determined with NAATs, but research in this area is ongoing. In addition, the major limitation of NAATs is the potential for false-positive results due to remnant nucleic acids, either from contamination or dead organisms; this property limits the utility of NAATs for immediate post-treatment testing.
Point-of-Care NAAT
There are several point-of-care tests that can accurately diagnose gonococcal infections.
- Binx Health: In March 2021, the FDA approved the first point-of-care NAAT (Binx Health IO CT/NG Assay) for the diagnosis of urogenital chlamydia and gonorrhea.[47] This point-of-care test can be run on vaginal swabs obtained from women or on urine samples collected from men.[47] This assay can provide a result in approximately 30 minutes.[47] In a cross-sectional study, investigators evaluated this point-of-care NAAT for the diagnosis of chlamydia and gonorrhea using vaginal swabs obtained from 1,523 women and urine samples collected from 922 men.[48] For gonorrhea, the sensitivity estimates were 100.0% in women and 97.3% in men; the specificity estimates were 99.9% for women and 100.0% for men.[48] In addition, the investigators found that self-obtained vaginal swabs in women performed equivalent to clinician-collected vaginal swabs.[48]
- Visby Medical Sexual Health Test: In March 2023, the FDA provided clearance and CLIA waiver for the second-generation Visby Medical point-of-care sexual health test. This CLIA-waived point-of-care PCR assay provides results within 30 minutes. This palm-sized device is for single use on a self-collected vaginal swab in women and can accurately detect C. trachomatis, N. gonorrhoeae, and Trichomonas vaginalis.[49] In an evaluation of 1,555 women presenting at clinical sites, the assay performed with a sensitivity of 97·4% and specificity of 99·4% for N.gonorrhoeae.[49]
At-Home Sample Collection for NAAT
In November 2023, the FDA granted marketing authorization for the first at-home sample collection kit (Simple 2 Test) for the diagnosis of urogenital chlamydia and gonorrhea in persons 18 years of age and older.[50,51] The collection kits include materials for persons to self-collect a vaginal swab or a penile urine sample to be shipped to a clinical laboratory to use the Aptima Combo 2 assay (NAAT).[52] The test is available over the counter for use without the supervision of a health care provider. In a cross-sectional study, investigators evaluated this test for the diagnosis of chlamydia and gonorrhea using vaginal swabs obtained from 1,258 women and urine samples collected from 1,797 men.[52] For gonorrhea diagnosis in women, the sensitivity and specificity estimates for vaginal swabs were 97.7% and 99.6%, respectively. For gonorrhea diagnosis in men, the sensitivity and specificity estimates for urine samples were 98.7% and 99.7% in men.[52] The presence of some brands of hand soap and hand sanitizer caused false-negative gonorrhea results in urine samples from men.
Gram Stain
The use of Gram stain is a nonculture test that can make a presumptive diagnosis of gonorrhea. In the clinical setting, a Gram stain to detect N. gonorrhoeae is most often performed on a male with purulent urethral discharge. A Gram stain on a specimen positive for N. gonorrhoeae shows polymorphonuclear leukocytes with intracellular gram-negative diplococci (Figure 9). A Gram stain, when using proper laboratory techniques, has greater than 95% sensitivity and greater than 99% specificity for diagnosing symptomatic male gonococcal urethritis.[43] Thus, the Gram stain is considered reliable both to diagnose and to exclude gonococcal urethritis in symptomatic men.[25] The sensitivity of a Gram stain is lower for males with asymptomatic urethral infection and thus not considered adequate to rule out infection in asymptomatic men.[25] Performing a Gram stain is not recommended on endocervical, pharyngeal, or rectal specimens due to poor sensitivity.[25]
Culture
Obtaining a bacterial culture is the historical standard for detecting N. gonorrhoeae. It has several advantages over nonculture tests, including low cost, use for a variety of specimen sites, and antimicrobial susceptibility testing if N. gonorrhoeae is isolated from the specimen. Despite having some advantages, culture is not as sensitive as NAAT and is more laboratory intensive, which has led to infrequent use in modern practice. Optimal growth of N. gonorrhoeae requires nutritional supplementation, such as with Thayer-Martin media. At present, culture is primarily used for antimicrobial resistance surveillance.[25]
Diagnosis in Cases of Possible Sexual Abuse/Assault
In cases of suspected sexual abuse or assault, the legal standard is to obtain culture samples combined with additional tests in an attempt to identify STIs, including N. gonorrhoeae.[25] Due to the legal complexity of these cases, it is imperative that all positive specimens be retained for additional confirmatory testing. In adults, NAATs are preferred for the diagnostic evaluation of sexual assault, regardless of whether penetration occurred during the assault.[] In evaluating children with suspected sexual abuse, either culture or NAAT can be used to detect N. gonorrhoeaeand C. trachomatis.[54] In this setting, making a diagnosis of an STI in a child has tremendous implications. When using a NAAT to detect N. gonorrhoeae, there is the possibility of cross-reaction with nongonococcal Neisseria species and other commensals.[54] Accordingly, if a NAAT is used for evaluation, it is important to include expert consultation in this process and ensure that the NAAT has been Clinical Laboratory Improvement Amendments (CLIA)-validated and FDA-cleared.[55] Further, it is important to ensure the results are appropriately interpreted. Any initial testing on a specimen that generates a positive result should have confirmatory testing, either by retesting the original specimen or obtaining a new specimen from the same site from where the original sample was obtained.[54] A Gram stain is inadequate for evaluating prepubertal children for gonorrhea and should not be used to diagnose or exclude gonorrhea.
Special Diagnostic Considerations with Disseminated Gonococcal Infection
When evaluating persons with suspected disseminated gonococcal infection, diagnostic testing should consist of (1) obtaining and ordering NAAT and culture for specimens obtained from all applicable urogenital and extragenital mucosal sites, (2) ordering culture for all specimens obtained from disseminated sites of infection (e.g., skin, synovial fluid, blood, or cerebrospinal fluid), and (3) performing antimicrobial susceptibility testing on all N. gonorrhoeae isolates obtained in culture specimens.
Reporting Requirements
Laws and regulations in all states require clinicians, laboratories, or both to report persons diagnosed with gonorrhea to local public health authorities. Reporting can be done by medical providers, laboratories, or both.
Screening for Gonococcal Infection
Routine screening for gonococcal infection in persons at increased risk is recommended in order to decrease morbidity and reduce the burden of disease in the community.[25,56] Testing samples should be obtained from the anatomic sites of sexual exposure. The following summarizes recommendations for gonorrhea screening in the 2021 STI Treatment Guidelines and [Guidelines] Doxy PEP Guidelines.[25,56,57]
Women [25]
- Routine screening for gonorrhea is recommended for all sexually active women younger than 25 years of age.
- Sexually active women who are 25 years of age and older should have screening for gonorrhea if they are at increased risk (other STIs, a new sex partner, more than one sex partner, a sex partner with concurrent partners, a sex partner who has an STI, or a sex partner who is exchanging sex for money or drugs).
- Screening for pharyngeal and rectal gonorrhea screening can be considered in females based on reported sexual activities and exposure through shared clinical decision-making.
Women Who Have Sex with Women (WSW) and Women Who Have Sex with Women and Men [58]
- Gonorrhea screening recommendations for women who have sex only with women (WSW) and women who have sex with women and men (WSWM) are the same as outlined above for women who have sex with men.
Pregnant Women [56,59]
- Routine screening for gonorrhea should be performed on all pregnant women younger than 25 years of age and in older pregnant women if at increased risk for gonococcal infection (other STIs, a new sex partner, more than one sex partner, a sex partner with concurrent partners, a sex partner who has an STI, or a sex partner who is exchanging sex for money or drugs).
- Retesting for gonorrhea should be done during the third trimester for women under 25 years of age or those with an increased risk of acquiring gonorrhea during the pregnancy.
Men Who Have Sex Only with Women [56]
- For men who have sex only with women, routine screening for gonococcal infection is not recommended.
Men Who Have Sex with Men [56,60]
- All sexually active MSM should undergo screening for gonorrhea at least annually.
- Screening should consist of testing at sites of sexual contact (urethra, rectum, pharynx). The preferred tests (self-collected or provider-collected) are urine NAAT, rectal NAAT, and pharyngeal NAAT.
- More frequent screening at 3- to 6-month intervals is indicated for men who have sex with men if they have increased risk (e.g., persons taking HIV preexposure prophylaxis, individuals with HIV, persons with ongoing risk of acquiring chlamydia, and persons who have multiple sex partners or their sex partners have multiple partners).
Persons with HIV [56]
- All persons with HIV who are sexually active should have screening for gonorrhea at the initial HIV evaluation visit and at least annually thereafter.
- Some individuals may require more frequent screening depending on their sexual activity and local epidemiology for their region.
Persons Receiving HIV Preexposure Prophylaxis (HIV PrEP)
- Persons receiving HIV preexposure prophylaxis (HIV PrEP) should undergo routine gonorrhea screening at baseline. The frequency of gonorrhea screening for persons while they are receiving HIV PrEP varies as follows:
- Every 3 months for MSM taking oral HIV PrEP (tenofovir DF-emtricitabine or tenofovir alafenamide-emtricitabine)
- Every 4 months for MSM who are receiving long-acting injectable cabotegravir
- Every 6 months for men who have sex with women and women who have sex with men (with any HIV PrEP)
Persons Receiving Doxycycline Postexposure Prophylaxis (Doxy PEP) [57]
- Persons starting on doxycycline postexposure prophylaxis (doxy PEP) should undergo routine gonorrhea screening at baseline.
- The frequency of gonorrhea screening for persons while they are continuing on doxy PEP should be every 3 to 6 months.
Persons in Correctional Facilities [61]
- Routine opt-out screening for gonococcal infection should be offered at intake upon entering a correctional facility for women 35 years of age and younger and for men younger than 30 years of age.
Treatment
Treatment of Gonococcal Infections
The following summarizes gonococcal treatment recommendations in the 2021 STI Treatment Guidelines.[25] These recommendations depend on the site of the infection and whether chlamydia infection has been ruled out.[25]
Uncomplicated Infections of the Cervix, Urethra, and Rectum
For persons with uncomplicated gonococcal infections of the cervix, urethra, or rectum, the recommended treatment for persons who weigh less than 150 kg is a single intramuscular dose of ceftriaxone 500 mg; for persons who weigh 150 kg or more, the ceftriaxone dose should be increased to 1 gram.[25] If chlamydia infection has not been excluded, then treatment should include oral doxycycline 100 mg twice daily for 7 days (except for pregnant women).[25] For pregnant women, if chlamydia has not been excluded, oral azithromycin 1 gram should be used in place of doxycycline.[25] If ceftriaxone is not available, the two options are (1) intramuscular gentamicin 240 mg plus oral azithromycin 2 grams or (2) oral cefixime 800 mg.[25]
Table 1. 2021 STI Treatment Guidelines: Gonococcal InfectionsTreatment of Uncomplicated Gonococcal Infection of the Cervix, Urethra, or RectumRecommended Regimen if Chlamydial Infection ExcludedCeftriaxone500 mg* IM in a single dose for persons weighing <150 kgCeftriaxone
Tradename:RocephinNote: *For persons weighing ≥150 kg, ceftriaxone 1 g IM should be administered.Recommended Regimen if Chlamydial Infection Has Not Been ExcludedCeftriaxone500 mg* IM in a single dose for persons weighing <150 kgCeftriaxone
Tradename:RocephinDoxycycline100 mg orally twice daily for 7 days+Doxycycline
Tradename:Doryx, VibramycinDuring pregnancy, oral azithromycin 1 gram in a single dose is recommended to treat chlamydia.Note: *For persons weighing ≥150 kg, ceftriaxone 1 g IM should be administered.Alternative Regimen if Ceftriaxone is Not AvailableGentamicin240 mg IM in a single doseGentamicin
Tradename:GaramycinAzithromycin2 g orally in a single dose+Azithromycin
Tradename:ZithromaxAlternative Regimen if Ceftriaxone is Not AvailableCefixime800 mg orally in a single doseCefixime
Tradename:SupraxNote: If treating with cefixime, and chlamydial infection has not been excluded, providers should treat for chlamydia with doxycycline 100 mg orally twice daily for 7 days. During pregnancy, oral azithromycin 1 g in a single dose is recommended to treat chlamydia.Source: Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. Gonococcal infections. MMWR Recomm Rep. 2021;70(No. RR-4):1-187. [2021 STI Treatment Guidelines]Check-on-Learning QuestionWhich one of the following is the recommended regimen for treatment for this 17-year-old male?Check
-On-
Learning
QuestionA 17-year-old male has a presumptive diagnosis of gonococcal urethritis based on a Gram stain of a urethral discharge specimen that shows multiple gram-negative intracellular diplococci. Nucleic acid amplification testing (NAAT) for Neisseria gonorrhoeae and Chlamydia trachomatis is sent. He denies any history of drug allergies.Which one of the following is the recommended regimen for treatment for this 17-year-old male?
Uncomplicated Infections of the Pharynx
Gonococcal infections of the pharynx are more difficult to eradicate than infections at urogenital and anorectal sites. The recommended treatment for pharyngeal gonorrhea for persons who weigh less than 150 kg is a single intramuscular dose of ceftriaxone 500 mg; for persons who weigh 150 kg or more, the ceftriaxone dose should be increased to 1 gram.[25] Note there are no reliable alternative regimens for the treatment of pharyngeal gonorrhea.[25] If chlamydia infection was also identified when the pharyngeal testing was performed, then treatment should include oral doxycycline 100 mg twice daily for 7 days (except for pregnant women).[25] If treatment for chlamydia pharyngeal infection is indicated and the patient is a woman who is pregnant, oral azithromycin (1 gram single-dose treatment) should be used in place of doxycycline. Concomitant treatment for pharyngeal chlamydial infection is not indicated if testing for pharyngeal chlamydia was not performed or if the testing for chlamydia was negative.[25]
Table 2. 2021 STI Treatment Guidelines: Gonococcal InfectionsTreatment of Uncomplicated Gonococcal Infection of the PharynxRecommended RegimenCeftriaxone500 mg* IM in a single dose for persons weighing <150 kgCeftriaxone
Tradename:RocephinIf chlamydial infection is identified when pharyngeal gonorrhea testing is performed, treat with doxycycline 100 mg orally 2 times a day for 7 days; women who are pregnant should receive azithromycin 1 g orally in a single dose (instead of doxycycline).Note: *For persons weighing ≥150 kg, ceftriaxone 1 g IM should be administered.No reliable alternative treatments are available for pharyngeal gonorrhea. For persons with a history of a beta-lactam allergy, a thorough assessment of the reaction is recommended. For persons with an anaphylactic or other severe reaction (e.g., Stevens Johnson syndrome) to ceftriaxone, consult an infectious disease specialist for an alternative treatment recommendation.Source: Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. Gonococcal infections. MMWR Recomm Rep. 2021;70(No. RR-4):1-187. [2021 STI Treatment Guidelines]Conjunctivitis
In the only published study of the treatment of gonococcal conjunctivitis among adults, all 12 study participants responded to a single 1-gram intramuscular injection of ceftriaxone.[62] Based on this study, the recommended treatment for gonococcal conjunctivitis is a single ceftriaxone 1-gram intramuscular dose.[25] In addition, a one-time lavage of the infected eye with saline should be considered.[25]
Table 3. 2021 STI Treatment Guidelines: Gonococcal InfectionsTreatment of Gonococcal Conjunctivitis Among Adolescents and AdultsRecommended RegimenCeftriaxone1 g IM in a single doseCeftriaxone
Tradename:RocephinProviders should consider one-time lavage of the infected eye with saline solution.Source: Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. Gonococcal infections. MMWR Recomm Rep. 2021;70(No. RR-4):1-187. [2021 STI Treatment Guidelines]Disseminated Gonococcal Infection
Persons with disseminated gonococcal infection (DGI) can develop severe sequelae and complications. Therefore, in addition to antimicrobial therapy, management should include hospitalization and consultation with an infectious diseases specialist.[25]
Disseminated Infection With Gonococcal-Related Arthritis and Arthritis-Dermatitis Syndrome
Disseminated gonococcal infection is often complicated by joint complications (e.g., polyarthralgia, tenosynovitis, or oligoarticular septic arthritis) and by dermatologic manifestations (e.g., pustular lesions and petechiae).[38,63,64] The following summarizes treatment of disseminated gonococcal infection, including with arthritis and/or dermatitis complications, consists of initial antimicrobial therapy with intramuscular or intravenous ceftriaxone 1 gram every 24 hours; if chlamydia has not been excluded, then treatment of chlamydia with doxycycline 100 mg twice daily should be given. The first dose of ceftriaxone should be given after promptly obtaining appropriate diagnostic samples. The duration of therapy for disseminated gonococcal infection with arthritis-dermatitis syndrome is at least 7 days, and the ceftriaxone can transition to oral therapy if the patient is clinically improved and antimicrobial sensitivity testing shows an effective oral choice.[25] If meningitis or endocarditis is diagnosed, then treatment should be changed to that recommended in the next section (Disseminated Infection with Meningitis and/or Endocarditis).[25]
Table 4. 2021 STI Treatment Guidelines: Gonococcal InfectionsTreatment of Disseminated Gonococcal Infection (DGI): Arthritis and Arthritis-Dermatitis SyndromeRecommended RegimenCeftriaxone1 g IM or IV every 24 hoursCeftriaxone
Tradename:RocephinNote: If chlamydial infection has not been excluded, providers should treat for chlamydia with doxycycline 100 mg orally 2 times/day for 7 days.Alternative RegimensCefotaxime1 gram IV every 8 hoursCefotaxime
Tradename:ClaforanNote: If chlamydial infection has not been excluded, providers should treat for chlamydia with doxycycline 100 mg orally 2 times/day for 7 days.Alternative RegimensCeftizoxime1 g IV every 8 hoursCeftizoxime
Tradename:CefizoxNote: If chlamydial infection has not been excluded, providers should treat for chlamydia with doxycycline 100 mg orally 2 times/day for 7 days.When treating for the arthritis-dermatitis syndrome, the provider can switch to an oral agent guided by antimicrobial susceptibility testing 24–48 hours after substantial clinical improvement, for a total treatment course of at least 7 days.Source: Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. Gonococcal infections. MMWR Recomm Rep. 2021;70(No. RR-4):1-187. [2021 STI Treatment Guidelines]Disseminated Infection With Meningitis and/or Endocarditis
For persons diagnosed with DGI and meningitis, parenteral therapy requires a higher dose of ceftriaxone, and therapy should continue for at least 10 to 14 days; with endocarditis, parenteral therapy should be given for at least 4 weeks.[25]
Table 5. 2021 STI Treatment Guidelines: Gonococcal InfectionsTreatment of Disseminated Gonococcal Infection: Meningitis and EndocarditisRecommended RegimenCeftriaxone1–2 g IV every 24 hoursCeftriaxone
Tradename:RocephinNote: If chlamydial infection has not been excluded, providers should treat for chlamydia with doxycycline 100 mg orally 2 times a day for 7 days.No recent studies have been published regarding treatment of DGI involving the CNS or cardiovascular system. The duration of treatment for DGI in these situations has not been systematically studied and should be determined in consultation with an infectious disease specialist. Treatment for DGI should be guided by the results of antimicrobial susceptibility testing. Length of treatment should be determined based on clinical presentation. Therapy for meningitis should be continued with recommended parenteral therapy for 10–14 days. Parenteral antimicrobial therapy for endocarditis should be administered for >4 weeks.Source: Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. Gonococcal infections. MMWR Recomm Rep. 2021;70(No. RR-4):1-187. [2021 STI Treatment Guidelines]Allergy to Penicillin or Cephalosporin
For persons who report a penicillin or cephalosporin allergy, it is important to determine the severity of the reaction, if the reaction was consistent with an IgE-mediated reaction, and whether the reaction occurred within the prior 10 years. Among all persons who self-reported a history of an allergic reaction to penicillin or another beta-lactam antibiotic, only 7.1% had a positive objective test that confirmed the penicillin allergy.[65] In addition, among persons with a history of penicillin allergy, fewer than 1.0% will have an allergic reaction to a third-generation cephalosporin, such as ceftriaxone or cefixime.[66] For persons who have a documented or strongly suspected IgE-mediated allergic reaction to penicillin or cephalosporins, one option is to administer dual treatment with a single dose of intramuscular gentamicin 240 mg plus a single dose of oral azithromycin 2 grams.[8,25,67] This regimen, however, is not recommended for the treatment of pharyngeal gonorrhea due to its low efficacy. Some individuals with a penicillin or cephalosporin allergy may require expert consultation with an allergy specialist, especially those with a history of an Ig-E mediated allergic reaction in the prior 10 years.
Gonococcal Infections in Pregnancy
Pregnant women with N. gonorrhoeae should be treated with a single intramuscular dose of ceftriaxone 500 mg, with the addition of a single dose of azithromycin 1.0 g orally if chlamydia infection is not ruled out.[3] Pregnant women should not be treated with any fluoroquinolone or tetracycline drugs. Because spectinomycin is not available in the United States, pregnant women who cannot tolerate a cephalosporin should be evaluated by an infectious diseases specialist.
Check-on-Learning QuestionWhich one of the following regimens should be given to treat this pregnant woman with cervicitis?Check
-On-
Learning
QuestionA 25-year-old pregnant woman is diagnosed with cervicitis at 20 weeks gestation. She reports a new vaginal discharge and dysuria. A sample of the purulent discharge is sent for nucleic acid amplification testing (NAAT), and the test is positive for gonorrhea but negative for chlamydia. She reports no other symptoms, and she has no drug allergies.Which one of the following regimens should be given to treat this pregnant woman with cervicitis?
Management of Antibiotic-Resistant Gonorrhea
Although there are no confirmed cases of treatment failure due to cephalosporin-resistant N. gonorrhoeae in the United States, the gradual upward trend of MICs documented by the United States Gonococcal Isolate Surveillance Project (GISP) remains worrisome.[68,69,70,71] Criteria for resistance to cefixime and ceftriaxone have not been defined by the Clinical and Laboratory Standards Institute (CLSI), but isolates with cefixime or ceftriaxone MICs equal to or greater than 0.5 μg/mL are considered to have decreased susceptibility. Only five isolates with ceftriaxone MIC equal to or greater than 0.5 μg/mL have been reported during the history of the GISP. In 2019, one isolate from Nevada was reported with a ceftriaxone MIC of 1.0 μg/mL.[7] Notably, isolates with high-level cefixime and ceftriaxone MICs (cefixime MICs 1.5 to 8 μg/mL and ceftriaxone MICs 1.5 to 4 μg/mL) have been identified in Japan, France, and Spain.[72,73,74,75]
Defining Gonococcal Treatment Failure
Cephalosporin treatment failure should be suspected in persons who meet the following criteria: (1) they have received appropriate treatment for gonorrhea; (2) they report no sexual contact during the post-treatment follow-up period; and (3) they remain symptomatic (symptoms do not resolve within 3 to 5 days of receiving treatment), or they have a positive test-of-cure (a positive culture greater than 72 hours after treatment or a positive NAAT greater than 7 days after treatment).[25] Risk factors for treatment failure due to resistant organisms include multiple prior treatment courses for gonorrhea, international travel, or pharyngeal disease.
Management of Suspected Gonococcal Treatment Failure
In the United States, gonococcal infections identified after treatment with ceftriaxone usually result from reinfection rather than treatment failure.[76,77,78] Persons with persistent infection following treatment and who deny any post-treatment sexual contact should undergo repeat testing and evaluation for N. gonorrhoeae infection that ideally includes culture evaluation.[25] Detection of N. gonorrhoeae infection in a person with suspected cephalosporin treatment failure should result in the following: (1) culture and susceptibility testing of all relevant clinical specimens; (2) guidance from expert opinion for clinical management (through the STD Clinical Consultation Network, a local STD/HIV Prevention Training Center clinical expert, the CDC, or an infectious diseases specialist); and (3) generation of a case report to the CDC through state and local public health authorities.[25] In cases of suspected cephalosporin treatment failure, isolates that grow N. gonorrhoeae should be saved and sent to the CDC through state public health laboratory mechanisms. Health departments should prioritize notification and culture evaluation for sex partner(s) of persons with N. gonorrhoeae infection who have suspected cephalosporin treatment failure and/or gonococcal isolates that demonstrate decreased susceptibility to cephalosporins.
- Initial Approach to Suspected Treatment Failure: Persons with suspected cephalosporin treatment failure should first receive retreatment with a single dose of intramuscular ceftriaxone 500 mg, with doxycycline if chlamydial infection exists. This initial approach is recommended in the United States because most suspected treatment failures result from reinfection.
- Antimicrobial Options for Likely Treatment Failure: For individuals considered to have a high likelihood of true treatment failure, especially those with a documented elevated cephalosporin MIC for N. gonorrhoeae, the suggested option to consider is single-dose oral therapy with azithromycin 2 grams plus a single intramuscular injection of a 240 mg dose of gentamicin.
- Investigational Therapy for N. gonorrhoeae: Several antimicrobials under investigation have shown promise in the treatment of gonorrhea in phase 2 trials, including single-dose oral gepotidacin and single-dose oral zoliflodacin.[79,80] Phase 3 trials are completed, with results pending. These two agents are now under study in phase 3 trials, and both may have a future role in treating drug-resistant gonorrhea and gonorrhea in persons with serious penicillin or cephalosporin allergy. Two oral agents—solithromycin and delafloxacin—showed promising early results, but phase 3 studies have been disappointing, and these agents are not likely to have a clinical role in the treatment of gonorrhea.[81,82,83,84]
Follow-Up
Due to the significant risk of reinfection in persons diagnosed with gonorrhea, all persons diagnosed with gonorrhea should have repeat testing in 3 months at the anatomic site of exposure, regardless of whether they have symptoms; this is considered a test for reinfection, not a test-of-cure.[25] For persons with uncomplicated gonococcal infections of the cervix, urethra, or rectum, a routine test-of-cure at 7 to 14 days post-treatment is not recommended.[25] For persons with pharyngeal gonorrhea, however, a routine test-of-cure (using either culture or NAAT) is recommended 7 to 14 days after completing treatment, regardless of the treatment regimen.[25] Because nucleic acid tests frequently remain positive for 7–10 days after treatment, many experts recommend doing the follow-up test-of-cure on post-treatment day 14 (or as near to post-treatment day 14 as possible). For these individuals, if the test-of-cure NAAT is positive, an effort should be made to perform a confirmatory culture before retreatment; all positive cultures for test-of-cure should undergo antimicrobial susceptibility testing.[56] For persons with suspected cephalosporin-resistant gonorrhea who receive retreatment, a test-of-cure at relevant clinical sites should be obtained 7 to 14 days after retreatment; culture is the recommended test, preferably with simultaneous NAAT and susceptibility testing of N. gonorrhoeae if isolated.[25]
Management of Sex Partners
Evaluation and Treatment of Sex Partners
All recent sex partners (within the 60 days preceding the onset of symptoms or gonorrhea diagnosis) should be referred for evaluation, testing, and presumptive treatment of gonorrhea. If the most recent contact with a sex partner occurred more than 60 days preceding the onset of symptoms or gonorrhea diagnosis, that partner should be referred for evaluation and treatment. Sex partners who are treated should also be instructed to abstain from sexual activity for 7 days after they have completed antimicrobial treatment.
Expedited Partner Therapy
In settings where prompt referral and treatment are unavailable or impractical, medical providers can consider expedited partner therapy.[25,85] This entails providing appropriate antibiotics as well as educational information for the partner. These written materials should include notification that partner(s) have been exposed, information about the importance of treatment, signs and symptoms of potential complications, as well as possible therapy-related allergic reactions and adverse effects.[25]
- Recommended Regimen: The expedited partner therapy regimen for sex partners of patients with N. gonorrhoeae infection is oral cefixime 800 mg with delivery of the prescription to the partner by either the person diagnosed with gonorrhea, a disease investigation specialist, or a collaborating pharmacy as permitted by law; if concurrent chlamydia was not excluded in the source individual who was diagnosed with gonorrhea, then the expedited partner therapy should include oral doxycycline 100 mg for 7 days, except for pregnant women; if there is concern regarding the partner taking multidose therapy for chlamydia, then azithromycin 1-gram orally as a single dose can be given.[25,86]
- State-by-State Legal Status: It is essential to check with one’s state health department to clarify the policies, as the use of expedited partner therapy is not legal in all states. The CDC maintains an updated information page, Legal Status of Expedited Partner Therapy, that identifies the legal status of expedited partner therapy in each state in the United States, as well as providing links to each state for more detailed state policies.
- Follow-Up After Expedited Partner Therapy: Provision of expedited partner therapy alone is not sufficient, and each partner should ideally be seen in follow-up for repeat testing to check for reinfection. Although offering expedited partner therapy to female partners is acceptable, this approach may result in the undertreatment of pelvic inflammatory disease.
- Contraindications for Expedited Partner Therapy: The use of expedited partner therapy for gonorrhea is contraindicated in a female partner who has current signs or symptoms that are suggestive of PID. Female partners who have current signs and symptoms suggestive of PID should undergo prompt evaluation by a health care provider. In addition, the use of expedited partner therapy should not be considered a routine partner management strategy in MSM with gonorrhea for several reasons, including the high risk for coexisting infections (especially HIV and syphilis), inadequate data regarding the efficacy of expedited partner therapy in this patient population, and concerns regarding the increased proportion of gonococcal isolates among MSM with reduced susceptibility to cefixime.
Counseling and Education
The following summarizes key counseling messages for persons diagnosed with gonococcal infection.
- Resuming Sexual Activity: Persons treated for gonococcal infection should receive instructions to abstain from sexual activity until all the following criteria are met: (1) at least 7 days have elapsed since completing treatment, (2) any genitourinary symptoms have resolved, and (3) if they are planning to resume sexual activity with a recent sex partner, the sex partner should have received appropriate treatment for gonorrhea and at least 7 days have elapsed since the partner was treated.
- Partner Notification: It is extremely important that persons treated for gonococcal infection understand the importance of partner notification (for all sex partners in the prior 60 days). Partner notification with evaluation and treatment can markedly reduce the spread of STIs in the community, and it also reduces the likelihood of reinfection for the person diagnosed with gonorrhea.
- Follow-Up Testing: It is important that all persons treated for gonorrhea have a follow-up visit in approximately 3 months for repeat STI testing. The purpose of this 3-month visit is to test for reinfection with gonorrhea, as well as to test for other STIs that could have been acquired in the 3-month post-treatment time frame.
- STI Prevention: At the time a person is receiving treatment for an STI, it is appropriate to provide counseling messages on how to prevent STIs in the future (e.g., limiting the number of sex partners and consistently using condoms).
Prevention
Doxycycline Post-Exposure Prophylaxis for Prevention of STIs
Doxycycline administered as 200 mg once within 72 hours after a condomless sexual encounter, can help prevent bacterial STIs, including chlamydia, syphilis, and gonorrhea. The use of doxycycline post-exposure prophylaxis (doxy PEP) has been shown to be effective for men who have sex with men in several randomized trials.[87,88,89] One clinical trial using doxy PEP for women in Africa has been completed, and it did not show benefit in reducing STIs.[90] In 2024, the CDC published Clinical Guidelines on the Use of Doxycycline Post-exposure Prophylaxis for Bacterial STI Prevention.[57] These guidelines do not recommend doxy PEP for women. The following will focus on the impact of doxy PEP on preventing gonococcal infections.
Doxycycline Post-Exposure Prophylaxis for Prevention of Gonorrhea
Data are mixed on the efficacy of doxy PEP for the prevention of gonococcal infections. In the DoxyPEP randomized trial in the United States, which was conducted in San Francisco and Seattle during 2020-2022, the use of doxy PEP, as compared to standard of care, led to an approximate 55% reduction in incident gonorrhea, including a major reduction in pharyngeal gonorrhea.[87] In addition, the quarterly incidence of new gonorrhea infections was significantly reduced in both main study arms—people without HIV who were receiving HIV PrEP and in those with HIV.[87] In the French IPERGAY-DoxyPEP study, which was conducted during 2015-2016, the use of doxy PEP among 232 MSM who were receiving HIV PrEP led to no significant changes in the occurrence of a new episode of gonorrhea, as compared to standard of care.[89] In another randomized trial involving MSM (the French ANRS DOXYVAC), there was moderate efficacy of doxy PEP against gonorrhea (33% reduction).[88] The most plausible reason for the discrepant findings between the United States and French trials is the difference in background prevalence of tetracycline resistance (roughly 27% in the United States versus 56% in France).[87,91] Given conflicting results thus far, more studies are needed regarding the efficacy of doxy PEP for the prevention of gonorrhea.
Meningococcal B Vaccine as Potential Prevention of Gonorrhea
Observational data have suggested reductions in gonorrhea among individuals who received outer membrane vesicle-based meningococcal vaccines, including the meningococcal group B vaccine/MenB-4C, a recombinant protein-based Neisseria meningitidis group B meningococcal vaccine that is licensed for use in the United States.[92,93,94] The 4C designation in the MenB-4C vaccine refers to the four main N. meningitidis recombinant antigens: Factor H-binding protein (FHbp), Neisserial adhesin A (NadA), Neisserial Heparin-Binding Antigen (NHBA), and Outer Membrane Vesicles OMV).[95] Vaccine-related cross-protection against gonorrhea is thought to arise from the 80-90% genetic sequence homology between the N. gonorrhoeae proteins and the OMV and NHBA antigens in the MenB-4C vaccine.[96,97] The antigens in the other licensed meningococcal B vaccine (MenB-FHbp) do not provide cross-protection against N. gonorrhoeae.[98] Similarly, the meningococcal pentavalent vaccine (MenACWY-TT/MenB-FHbp) does not have cross-protection with N. gonorrhoeae since the Men B antigens used in this vaccine are MenB-FHbp.[98] The ANRS DOXYVAC trial randomized MSM taking HIV PrEP to assess whether 2 doses of MenB-4C vaccination, given 2 months apart, versus placebo, would reduce the incidence of gonorrhea in MSM, but there was not a statistically significant reduction in gonorrhea incidence.[88] Randomized trials using 4CMenB for the prevention of gonorrhea are ongoing, including the HPTN 107 study, a randomized, placebo-controlled multi-site trial among men and women considered disproportionately vulnerable to N. gonorrhoeae infection.
Summary Points
- In the United States, rates of gonococcal infection have increased overall since 2010. The age group with the highest gonorrhea rates is persons 20-24 years of age.
- Gonococcal antimicrobial resistance to ceftriaxone and cefixime remains low in the United States. Antimicrobial resistance to azithromycin has increased in recent years. Resistance rates are high with penicillin, tetracycline, and ciprofloxacin.
- Gonorrhea is associated with increased susceptibility to HIV acquisition as well as an increased risk of HIV transmission.
- Neisseria gonorrhoeae can cause a wide array of urogenital, pharyngeal, and rectal symptoms as well as serious complications, such as pelvic inflammatory disease, tubal infertility, ectopic pregnancy, neonatal conjunctivitis, and rarely, disseminated infection.
- Screening for gonorrhea is recommended in sexually active women under 25 years of age and in other persons at increased risk of acquiring N. gonorrhoeae.
- The NAAT is the preferred test for screening and diagnosing gonorrhea in both men and women. Culture is now primarily used if there is a concern for antimicrobial-resistant gonococcal infection.
- Therapy with intramuscular ceftriaxone 500 mg is recommended in all persons (including pregnant women) with uncomplicated gonococcal infections of the cervix, urethra, rectum, or pharynx. If chlamydia infection has not been excluded, then concomitant treatment for chlamydia should be given with doxycycline 100 mg twice daily for 7 days; for pregnant women, a single 1-gram dose of oral azithromycin should be used instead of doxycycline to treat chlamydia.
- A test-of-cure is not routinely recommended after treatment of uncomplicated infections of the cervix, urethra, and rectum, but it should be performed in all persons at 7 to 14 days following the treatment of pharyngeal gonorrhea.
- Most cases of suspected treatment failures represent reinfection with N. gonorrhoeae, but if true cephalosporin treatment failure is suspected, clinicians should perform culture and sensitivity testing and seek expert consultation.
- Persons who are diagnosed with gonorrhea should receive counseling about the nature of the infection, the importance of partner notification, when they can resume sexual activity, and how they can reduce their risk of acquiring STIs in the future.
Check-On-Learning Questions Display Options
These quick questions are meant to keep you on track and check your understanding. They appear throughout the core concepts and are listed here for you to review.Check-on-Learning QuestionWhich one of the following is the recommended regimen for treatment for this 17-year-old male?Check
-On-
Learning
QuestionA 17-year-old male has a presumptive diagnosis of gonococcal urethritis based on a Gram stain of a urethral discharge specimen that shows multiple gram-negative intracellular diplococci. Nucleic acid amplification testing (NAAT) for Neisseria gonorrhoeae and Chlamydia trachomatis is sent. He denies any history of drug allergies.Which one of the following is the recommended regimen for treatment for this 17-year-old male?
Check-on-Learning QuestionWhich one of the following regimens should be given to treat this pregnant woman with cervicitis?Check
-On-
Learning
QuestionA 25-year-old pregnant woman is diagnosed with cervicitis at 20 weeks gestation. She reports a new vaginal discharge and dysuria. A sample of the purulent discharge is sent for nucleic acid amplification testing (NAAT), and the test is positive for gonorrhea but negative for chlamydia. She reports no other symptoms, and she has no drug allergies.Which one of the following regimens should be given to treat this pregnant woman with cervicitis?
Citations
- 1.Centers for Disease Control and Prevention. Sexually Transmitted Infections Surveillance, 2023. Atlanta: U.S. Department of Health and Human Services; November 2024.
- 2.Chesson HW, Spicknall IH, Bingham A, et al. The Estimated Direct Lifetime Medical Costs of Sexually Transmitted Infections Acquired in the United States in 2018. Sex Transm Dis. 2021;48:215-21.[PubMed Abstract] -
- 3.St Cyr S, Barbee L, Workowski KA, et al. Update to CDC's Treatment Guidelines for Gonococcal Infection, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1911-6.[PubMed Abstract] -
- 4.Kirkcaldy RD, Harvey A, Papp JR, et al. Neisseria gonorrhoeae Antimicrobial Susceptibility Surveillance - The Gonococcal Isolate Surveillance Project, 27 Sites, United States, 2014. MMWR Surveill Summ. 2016;65:1-19.[PubMed Abstract] -
- 5.Bolan GA, Sparling PF, Wasserheit JN. The emerging threat of untreatable gonococcal infection. N Engl J Med. 2012;366:485-7.[PubMed Abstract] -
- 6.Centers for Disease Control and Prevention. Gonococcal Isolate Surveillance Project (GISP) Profiles, 2022. April 2024.[CDC] -
- 7.Picker MA, Knoblock RJ, Hansen H, et al. Notes from the Field: First Case in the United States of Neisseria gonorrhoeae Harboring Emerging Mosaic penA60 Allele, Conferring Reduced Susceptibility to Cefixime and Ceftriaxone. MMWR Morb Mortal Wkly Rep. 2020;69:1876-77.[PubMed Abstract] -
- 8.Ross JDC, Brittain C, Cole M, et al. Gentamicin compared with ceftriaxone for the treatment of gonorrhoea (G-ToG): a randomised non-inferiority trial. Lancet. 2019;393:2511-20.[PubMed Abstract] -
- 9.Brown LB, Krysiak R, Kamanga G, et al. Neisseria gonorrhoeae antimicrobial susceptibility in Lilongwe, Malawi, 2007. Sex Transm Dis. 2010;37:169-72.[PubMed Abstract] -
- 10.Cohen MS, Cannon JG. Human experimentation with Neisseria gonorrhoeae: progress and goals. J Infect Dis. 1999;179 Suppl 2:S375-9.[PubMed Abstract] -
- 11.Quillin SJ, Seifert HS. Neisseria gonorrhoeae host adaptation and pathogenesis. Nat Rev Microbiol. 2018;16:226-40.[PubMed Abstract] -
- 12.Hill SA, Masters TL, Wachter J. Gonorrhea - an evolving disease of the new millennium. Microb Cell. 2016;3:371-89.[PubMed Abstract] -
- 13.Hooper RR, Reynolds GH, Jones OG, et al. Cohort study of venereal disease. I: the risk of gonorrhea transmission from infected women to men. Am J Epidemiol. 1978;108:136-44.[PubMed Abstract] -
- 14.Lin JS, Donegan SP, Heeren TC, et al. Transmission of Chlamydia trachomatis and Neisseria gonorrhoeae among men with urethritis and their female sex partners. J Infect Dis. 1998;178:1707-12.[PubMed Abstract] -
- 15.Bro-Jorgensen A, Jensen T. Gonococcal pharyngeal infections. Report of 110 cases. Br J Vener Dis. 1973;49:491-9.[PubMed Abstract] -
- 16.Sackel SG, Alpert S, Fiumara NJ, Donner A, Laughlin CA, McCormack WM. Orogenital contact and the isolation of Neisseria gonorrhoeae, Mycoplasma hominis, and Ureaplasma urealyticum from the pharynx. Sex Transm Dis. 1979;6:64-8.[PubMed Abstract] -
- 17.Barbee LA, Soge OO, Khosropour CM, et al. The Duration of Pharyngeal Gonorrhea: A Natural History Study. Clin Infect Dis. 2021;73:575-82.[PubMed Abstract] -
- 18.Templeton DJ, Jin F, McNally LP, et al. Prevalence, incidence and risk factors for pharyngeal gonorrhoea in a community-based HIV-negative cohort of homosexual men in Sydney, Australia. Sex Transm Infect. 2010;86:90-6.[PubMed Abstract] -
- 19.Cornelisse VJ, Bradshaw CS, Chow EPF, Williamson DA, Fairley CK. Oropharyngeal Gonorrhea in Absence of Urogenital Gonorrhea in Sexual Network of Male and Female Participants, Australia, 2018. Emerg Infect Dis. 2019;25:1373-6.[PubMed Abstract] -
- 20.Barbee LA, Khosropour CM, Dombrowski JC, Manhart LE, Golden MR. An estimate of the proportion of symptomatic gonococcal, chlamydial and non-gonococcal non-chlamydial urethritis attributable to oral sex among men who have sex with men: a case-control study. Sex Transm Infect. 2016;92:155-60.[PubMed Abstract] -
- 21.Bernstein KT, Stephens SC, Barry PM, et al. Chlamydia trachomatis and Neisseria gonorrhoeae transmission from the oropharynx to the urethra among men who have sex with men. Clin Infect Dis. 2009;49:1793-7.[PubMed Abstract] -
- 22.Marcus JL, Kohn RP, Barry PM, Philip SS, Bernstein KT. Chlamydia trachomatis and Neisseria gonorrhoeae transmission from the female oropharynx to the male urethra. Sex Transm Dis. 2011;38:372-3.[PubMed Abstract] -
- 23.Barbee LA, Khosropour CM, Dombrowksi JC, Golden MR. New human immunodeficiency virus diagnosis independently associated with rectal gonorrhea and chlamydia in men who have sex with men. Sex Transm Dis. 2017;44:385-9.[PubMed Abstract] -
- 24.Cohen MS, Hoffman IF, Royce RA, et al. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. AIDSCAP Malawi Research Group. Lancet. 1997;349:1868-73.[PubMed Abstract] -
- 25.Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. Gonococcal infections. MMWR Recomm Rep. 2021;70(No. RR-4):1-187.
- 26.Handsfield HH, Lipman TO, Harnisch JP, Tronca E, Holmes KK. Asymptomatic gonorrhea in men. Diagnosis, natural course, prevalence and significance. N Engl J Med. 1974;290:117-23.[PubMed Abstract] -
- 27.Harrison WO, Hooper RR, Wiesner PJ, et al. A trial of minocycline given after exposure to prevent gonorrhea. N Engl J Med. 1979;300:1074-8.[PubMed Abstract] -
- 28.Klausner JD, Kohn R, Kent C. Etiology of clinical proctitis among men who have sex with men. Clin Infect Dis. 2004;38:300-2.[PubMed Abstract] -
- 29.Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. Diseases characterized by urethritis and cervicitis. MMWR Recomm Rep. 2021;70(No. RR-4):1-187.
- 30.McCormack WM, Stumacher RJ, Johnson K, Donner A. Clinical spectrum of gonococcal infection in women. Lancet. 1977;1:1182-5.[PubMed Abstract] -
- 31.Chan PA, Robinette A, Montgomery M, et al. Extragenital infections caused by Chlamydia trachomatis and Neisseria gonorrhoeae: a review of the literature. Infect Dis Obstet Gynecol. 2016;2016:5758387.[PubMed Abstract] -
- 32.Brunham RC, Gottlieb SL, Paavonen J. Pelvic inflammatory disease. N Engl J Med. 2015;372:2039-48.[PubMed Abstract] -
- 33.Tice AW Jr, Rodriguez VL. Pharyngeal gonorrhea. JAMA. 1981;246:2717-9.[PubMed Abstract] -
- 34.Chow EPF, Chen MY, Williamson DA, et al. Oropharyngeal and genital gonorrhea infections among women and heterosexual men reporting sexual contact with partners With gonorrhea: implication for oropharyngeal testing of heterosexual gonorrhea contacts. Sex Transm Dis. 2019;46:743-7.[PubMed Abstract] -
- 35.Morris SR, Klausner JD, Buchbinder SP, et al. Prevalence and incidence of pharyngeal gonorrhea in a longitudinal sample of men who have sex with men: the EXPLORE study. Clin Infect Dis. 2006;43:1284-9.[PubMed Abstract] -
- 36.Balmelli C, Günthard HF. Gonococcal tonsillar infection--a case report and literature review. Infection. 2003;31:362-5.[PubMed Abstract] -
- 37.Wan WL, Farkas GC, May WN, Robin JB. The clinical characteristics and course of adult gonococcal conjunctivitis. Am J Ophthalmol. 1986;102:575-83.[PubMed Abstract] -
- 38.Bleich AT, Sheffield JS, Wendel GD Jr, Sigman A, Cunningham FG. Disseminated gonococcal infection in women. Obstet Gynecol. 2012;119:597-602.[PubMed Abstract] -
- 39.Crew PE, Abara WE, McCulley L, et al. Disseminated Gonococcal Infections in Patients Receiving Eculizumab: A Case Series. Clin Infect Dis. 2019;69:596-600.[PubMed Abstract] -
- 40.Kerle KK, Mascola JR, Miller TA. Disseminated gonococcal infection. Am Fam Physician. 1992;45:209-14.[PubMed Abstract] -
- 41.O'Brien JP, Goldenberg DL, Rice PA. Disseminated gonococcal infection: a prospective analysis of 49 patients and a review of pathophysiology and immune mechanisms. Medicine (Baltimore). 1983;62:395-406.[PubMed Abstract] -
- 42.Nettleton WD, Kent JB, Macomber K, et al. Notes from the field: ongoing cluster of highly related disseminated gonococcal infections - Southwest Michigan, 2019. MMWR Morb Mortal Wkly Rep. 2020;69:353-4.[PubMed Abstract] -
- 43.Centers for Disease Control and Prevention. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae--2014. MMWR Recomm Rep. 2014;63:1-19.[PubMed Abstract] -
- 44.Bachmann LH, Johnson RE, Cheng H, et al. Nucleic acid amplification tests for diagnosis of Neisseria gonorrhoeae and Chlamydia trachomatis rectal infections. J Clin Microbiol. 2010;48:1827-32.[PubMed Abstract] -
- 45.Bachmann LH, Johnson RE, Cheng H, Markowitz LE, Papp JR, Hook EW 3rd. Nucleic acid amplification tests for diagnosis of Neisseria gonorrhoeae oropharyngeal infections. J Clin Microbiol. 2009;47:902-7.[PubMed Abstract] -
- 46.U.S. Food and Drug Administration. FDA clears first diagnostic tests for extragenital testing for chlamydia and gonorrhea. FDA news release. May 23, 2019.[U.S. FDA] -
- 47.U.S. Food and Drug Administration: FDA News Release. FDA allows for first point-of-care Chlamydia and Gonorrhea test to be used in more near-patient care settings. FDA news release. March 30, 2021.[U.S. FDA] -
- 48.Van Der Pol B, Taylor SN, Mena L, et al. Evaluation of the Performance of a Point-of-Care Test for Chlamydia and Gonorrhea. JAMA Netw Open. 2020;3:e204819.[PubMed Abstract] -
- 49.Morris SR, Bristow CC, Wierzbicki MR, et al. Performance of a single-use, rapid, point-of-care PCR device for the detection of Neisseria gonorrhoeae, Chlamydia trachomatis, and Trichomonas vaginalis: a cross-sectional study. Lancet Infect Dis. 2021;21:668-76.[PubMed Abstract] -
- 50.U.S. Food and Drug Administration. FDA News Release. FDA Grants Marketing Authorization of First Test for Chlamydia and Gonorrhea with at-home Sample Collection[FDA] -
- 51.Kersh EN, Mena LA. At-Home Diagnostics Solutions for Chlamydia and Gonorrhea. JAMA. 2024;331:1701-2.[PubMed Abstract] -
- 52.Simple 2 Test Package Insert. For Detection of chlamydia and gonorrhea with: Simple 2 Urine Home Collection Kit (Penile) and Simple 2 Swab Home Collection Kit (Vaginal).
- 54.Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. Sexual assault and abuse and STIs: sexual assault or abuse of children. MMWR Recomm Rep. 2021;70(No. RR-4):1-187.
- 55.Brownell AD, Shapiro RA, Hammerschlag MR. Caution Is Required When Using Non-Food and Drug Administration-Cleared Assays to Diagnose Sexually Transmitted Infections in Children. J Pediatr. 2019;206:280-2.[PubMed Abstract] -
- 56.Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. Screening recommendations and considerations referenced in treatment guidelines and original sources. MMWR Recomm Rep. 2021;70(No. RR-4):1-187.
- 57.Bachmann LH, Barbee LA, Chan P, et al. CDC Clinical Guidelines on the Use of Doxycycline Postexposure Prophylaxis for Bacterial Sexually Transmitted Infection Prevention, United States, 2024. MMWR Recomm Rep. 2024;73:1-8.[PubMed Abstract] -
- 58.Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. Detection of STIs in special populations: women who have sex with women (WSW) and women who have sex with women and men (WSWM). MMWR Recomm Rep. 2021;70(No. RR-4):1-187.
- 59.Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. Detection of STIs in special populations: pregnant women. MMWR Recomm Rep. 2021;70(No. RR-4):1-187.
- 60.Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. Detection of STIs in special populations: men who have sex with men (MSM). MMWR Recomm Rep. 2021;70(No. RR-4):1-187.
- 61.Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. Detection of STIs in special populations: persons in correctional facilities. MMWR Recomm Rep. 2021;70(No. RR-4):1-187.
- 62.Haimovici R, Roussel TJ. Treatment of gonococcal conjunctivitis with single-dose intramuscular ceftriaxone. Am J Ophthalmol. 1989;107:511-4.[PubMed Abstract] -
- 63.Belkacem A, Caumes E, Ouanich J, et al. Changing patterns of disseminated gonococcal infection in France: cross-sectional data 2009-2011. Sex Transm Infect. 2013;89:613-5.[PubMed Abstract] -
- 64.Birrell JM, Gunathilake M, Singleton S, Williams S, Krause V. Characteristics and Impact of Disseminated Gonococcal Infection in the "Top End" of Australia. Am J Trop Med Hyg. 2019;101:753-60.[PubMed Abstract] -
- 65.Gadde J, Spence M, Wheeler B, Adkinson NF Jr. Clinical experience with penicillin skin testing in a large inner-city STD clinic. JAMA. 1993;270:2456-63.[PubMed Abstract] -
- 66.Novalbos A, Sastre J, Cuesta J, et al. Lack of allergic cross-reactivity to cephalosporins among patients allergic to penicillins. Clin Exp Allergy. 2001;31:438-43.[PubMed Abstract] -
- 67.Kirkcaldy RD, Weinstock HS, Moore PC, et al. The efficacy and safety of gentamicin plus azithromycin and gemifloxacin plus azithromycin as treatment of uncomplicated gonorrhea. Clin Infect Dis. 2014;59:1083-91.[PubMed Abstract] -
- 68.Centers for Disease Control and Prevention (CDC). Cephalosporin susceptibility among Neisseria gonorrhoeae isolates--United States, 2000-2010. MMWR Morb Mortal Wkly Rep. 2011;60:873-7.[PubMed Abstract] -
- 69.Centers for Disease Control and Prevention (CDC). Fluoroquinolone-resistance in Neisseria gonorrhoeae, Hawaii, 1999, and decreased susceptibility to azithromycin in N. gonorrhoeae, Missouri, 1999. MMWR Morb Mortal Wkly Rep. 2000;49:833-7.[PubMed Abstract] -
- 70.Centers for Disease Control and Prevention (CDC). Update to CDC's sexually transmitted diseases treatment guidelines, 2006: fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb Mortal Wkly Rep. 2007;56:332-6.[PubMed Abstract] -
- 71.Centers for Disease Control and Prevention. Increases in fluoroquinolone-resistant Neisseria gonorrhoeae among men who have sex with men--United States, 2003, and revised recommendations for gonorrhea treatment, 2004. MMWR Morb Mortal Wkly Rep. 2004;53:335-8.[PubMed Abstract] -
- 72.Cámara J, Serra J, Ayats J, et al. Molecular characterization of two high-level ceftriaxone-resistant Neisseria gonorrhoeae isolates detected in Catalonia, Spain. J Antimicrob Chemother. 2012;67:1858-60.[PubMed Abstract] -
- 73.Deguchi T, Yasuda M, Hatazaki K, et al. New Clinical Strain of Neisseria gonorrhoeae with Decreased Susceptibility to Ceftriaxone, Japan. Emerg Infect Dis. 2016;22:142-4.[PubMed Abstract] -
- 74.Ohnishi M, Golparian D, Shimuta K, et al. Is Neisseria gonorrhoeae initiating a future era of untreatable gonorrhea?: detailed characterization of the first strain with high-level resistance to ceftriaxone. Antimicrob Agents Chemother. 2011;55:3538-45.[PubMed Abstract] -
- 75.Unemo M, Golparian D, Nicholas R, Ohnishi M, Gallay A, Sednaoui P. High-level cefixime- and ceftriaxone-resistant Neisseria gonorrhoeae in France: novel penA mosaic allele in a successful international clone causes treatment failure. Antimicrob Agents Chemother. 2012;56:1273-80.[PubMed Abstract] -
- 76.Fung M, Scott KC, Kent CK, Klausner JD. Chlamydial and gonococcal reinfection among men: a systematic review of data to evaluate the need for retesting. Sex Transm Infect. 2007;83:304-9.[PubMed Abstract] -
- 77.Hosenfeld CB, Workowski KA, Berman S, et al. Repeat infection with Chlamydia and gonorrhea among females: a systematic review of the literature. Sex Transm Dis. 2009;36:478-89.[PubMed Abstract] -
- 78.Peterman TA, Tian LH, Metcalf CA, et al. High incidence of new sexually transmitted infections in the year following a sexually transmitted infection: a case for rescreening. Ann Intern Med. 2006;145:564-72.[PubMed Abstract] -
- 79.Taylor SN, Marrazzo J, Batteiger BE, et al. Single-Dose Zoliflodacin (ETX0914) for Treatment of Urogenital Gonorrhea. N Engl J Med. 2018;379:1835-45.[PubMed Abstract] -
- 80.Taylor SN, Morris DH, Avery AK, et al. Gepotidacin for the Treatment of Uncomplicated Urogenital Gonorrhea: A Phase 2, Randomized, Dose-Ranging, Single-Oral Dose Evaluation. Clin Infect Dis. 2018;67:504-512.[PubMed Abstract] -
- 81.Hook EW 3rd, Golden M, Jamieson BD, et al. A Phase 2 Trial of Oral Solithromycin 1200 mg or 1000 mg as Single-Dose Oral Therapy for Uncomplicated Gonorrhea. Clin Infect Dis. 2015;61:1043-8.[PubMed Abstract] -
- 82.Chen MY, McNulty A, Avery A, et al. Solithromycin versus ceftriaxone plus azithromycin for the treatment of uncomplicated genital gonorrhoea (SOLITAIRE-U): a randomised phase 3 non-inferiority trial. Lancet Infect Dis. 2019 Aug;19:833-42.[PubMed Abstract] -
- 83.Hook EW 3rd, Golden MR, Taylor SN, et al. Efficacy and Safety of Single-Dose Oral Delafloxacin Compared With Intramuscular Ceftriaxone for Uncomplicated Gonorrhea Treatment: An Open-Label, Noninferiority, Phase 3, Multicenter, Randomized Study. Sex Transm Dis. 2019;46:279-86.[PubMed Abstract] -
- 84.Soge OO, Salipante SJ, No D, Duffy E, Roberts MC. In Vitro Activity of Delafloxacin against Clinical Neisseria gonorrhoeae Isolates and Selection of Gonococcal Delafloxacin Resistance. Antimicrob Agents Chemother. 2016;60:3106-11.[PubMed Abstract] -
- 85.Golden MR, Whittington WL, Handsfield HH, et al. Effect of expedited treatment of sex partners on recurrent or persistent gonorrhea or chlamydial infection. N Engl J Med. 2005;352:676-85.[PubMed Abstract] -
- 86.Marrazzo JM, Martin DH. Management of women with cervicitis. Clin Infect Dis. 2007;44 Suppl 3:S102-10.[PubMed Abstract] -
- 87.Luetkemeyer AF, Donnell D, Dombrowski JC, et al. Postexposure Doxycycline to Prevent Bacterial Sexually Transmitted Infections. N Engl J Med. 2023;388:1296-1306.[PubMed Abstract] -
- 88.Molina JM, Bercot B, Assoumou L, et al. Doxycycline prophylaxis and meningococcal group B vaccine to prevent bacterial sexually transmitted infections in France (ANRS 174 DOXYVAC): a multicentre, open-label, randomised trial with a 2 × 2 factorial design. Lancet Infect Dis. 2024;24:1093-1104.[PubMed Abstract] -
- 89.Molina JM, Charreau I, Chidiac C, et al. Post-exposure prophylaxis with doxycycline to prevent sexually transmitted infections in men who have sex with men: an open-label randomised substudy of the ANRS IPERGAY trial. Lancet Infect Dis. 2018;18:308-317.[PubMed Abstract] -
- 90.Stewart J, Oware K, Donnell D, et al. Doxycycline Prophylaxis to Prevent Sexually Transmitted Infections in Women. N Engl J Med. 2023;389:2331-40.[PubMed Abstract] -
- 91.La Ruche G, Goubard A, Bercot B, Cambau E, Semaille C, Sednaoui P. Gonococcal infections and emergence of gonococcal decreased susceptibility to cephalosporins in France, 2001 to 2012. Euro Surveill. 2014;19:20885.[PubMed Abstract] -
- 92.Petousis-Harris H, Paynter J, Morgan J, et al. Effectiveness of a group B outer membrane vesicle meningococcal vaccine against gonorrhoea in New Zealand: a retrospective case-control study. Lancet. 2017 Sep 30;390:1603-1610.[PubMed Abstract] -
- 93.Ladhani SN, White PJ, Campbell H, et al. Use of a meningococcal group B vaccine (4CMenB) in populations at high risk of gonorrhoea in the UK. Lancet Infect Dis. 2024;24:e576-e583.[PubMed Abstract] -
- 94.Abara WE, Bernstein KT, Lewis FMT, et al. Effectiveness of a serogroup B outer membrane vesicle meningococcal vaccine against gonorrhoea: a retrospective observational study. Lancet Infect Dis. 2022;22:1021-9.[PubMed Abstract] -
- 95.Williams E, Seib KL, Fairley CK, et al. Neisseria gonorrhoeae vaccines: a contemporary overview. Clin Microbiol Rev. 2024;37:e0009423.[PubMed Abstract] -
- 96.Marjuki H, Topaz N, Joseph SJ, Gernert KM, Kersh EN, Wang X. Genetic Similarity of Gonococcal Homologs to Meningococcal Outer Membrane Proteins of Serogroup B Vaccine. mBio. 2019;10:e01668-19[PubMed Abstract] -
- 97.Ruiz García Y, Sohn WY, Seib KL, et al. Looking beyond meningococcal B with the 4CMenB vaccine: the Neisseria effect. NPJ Vaccines. 2021;6:130.[PubMed Abstract] -
- 98.Wang B, Mohammed H, Andraweera P, McMillan M, Marshall H. Vaccine effectiveness and impact of meningococcal vaccines against gonococcal infections: A systematic review and meta-analysis. J Infect. 2024;89:106225.[PubMed Abstract] -
Additional References
- Ahmed KA, Fox SJ, Frigas E, Park MA. Clinical outcome in the use of cephalosporins in pediatric patients with a history of penicillin allergy. Int Arch Allergy Immunol. 2012;158:405-10.[PubMed Abstract] -
- Beymer MR, Llata E, Stirland AM, et al. Evaluation of gonorrhea test of cure at 1 week in a Los Angeles community-based clinic serving men who have sex with men. Sex Transm Dis. 2014;41:595-600.[PubMed Abstract] -
- Bissessor M, Whiley DM, Fairley CK, et al. Persistence of Neisseria gonorrhoeae DNA following treatment for pharyngeal and rectal gonorrhea is influenced by antibiotic susceptibility and reinfection. Clin Infect Dis. 2015;60:557-63.[PubMed Abstract] -
- Chesson HW, Kent CK, Owusu-Edusei K Jr, Leichliter JS, Aral SO. Disparities in sexually transmitted disease rates across the "eight Americas". Sex Transm Dis. 2012;39:458-64.[PubMed Abstract] -
- Dombrowski JC, Donnell D, Grabow C, et al. Evidence-Informed Provision of Doxycycline Post-Exposure Prophylaxis for Prevention of Bacterial Sexually Transmitted Infections. Clin Infect Dis. 2024 Oct 30. Online ahead of print.[PubMed Abstract] -
- Goire N, Lahra MM, Chen M, et al. Molecular approaches to enhance surveillance of gonococcal antimicrobial resistance. Nat Rev Microbiol. 2014;12:223-9.[PubMed Abstract] -
- Lewis DA. New treatment options for Neisseria gonorrhoeae in the era of emerging antimicrobial resistance. Sex Health. 2019;16:449-56.[PubMed Abstract] -
- Newman LM, Berman SM. Epidemiology of STD disparities in African American communities. Sex Transm Dis. 2008;35:S4-12.[PubMed Abstract] -
- Unemo M, Seifert HS, Hook EW 3rd, Hawkes S, Ndowa F, Dillon JR. Gonorrhoea. Nat Rev Dis Primers. 2019;5:79.[PubMed Abstract] -
- Unemo M, Shafer WM. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: past, evolution, and future. Clin Microbiol Rev. 2014;27:587-613.[PubMed Abstract] -
- Wind CM, Schim van der Loeff MF, Unemo M, Schuurman R, van Dam AP, de Vries HJ. Test of Cure for Anogenital Gonorrhoea Using Modern RNA-Based and DNA-Based Nucleic Acid Amplification Tests: A Prospective Cohort Study. Clin Infect Dis. 2016;62:1348-55.[PubMed Abstract] -
- Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. Detection of STIs in special populations. MMWR Recomm Rep. 2021;70(No. RR-4):1-187.
- Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. Sexual assault and abuse and STIs. MMWR Recomm Rep. 2021;70(No. RR-4):1-187.
Figures
 Figure 1 (Image Series). Gonorrhea Reported Cases in the United StatesSource: Centers for Disease Control and Prevention. Sexually Transmitted Infections Surveillance, 2023. Atlanta: U.S. Department of Health and Human Services; November 2024.
Figure 1 (Image Series). Gonorrhea Reported Cases in the United StatesSource: Centers for Disease Control and Prevention. Sexually Transmitted Infections Surveillance, 2023. Atlanta: U.S. Department of Health and Human Services; November 2024. Figure 2 (Image Series). Gonococcal Isolate Surveillance Project (GISP), GISP 2010-2022Abbreviations: GISP = Gonococcal Isolate Surveillance Project (GISP)
Figure 2 (Image Series). Gonococcal Isolate Surveillance Project (GISP), GISP 2010-2022Abbreviations: GISP = Gonococcal Isolate Surveillance Project (GISP)
NOTE: In 2022, Cefixime 800 mg (0.1%) and Ceftriaxone 1 g (0.2%) each represented less than one percent of primary antimicrobial drugs used to treat gonorrhea among GISP participants and may not be visible in this figure.Source: Centers for Disease Control and Prevention. Gonococcal Isolate Surveillance Project (GISP) Profiles, 2022. Figure 2B. Neisseria gonorrhoeae—Percentage of Isolates with Elevated MICs to Azithromycin, Cefixime, and CeftriaxoneAbbreviations: GISP = Gonococcal Isolate Surveillance Project; MICs = minimum inhibitory concentrations;
Figure 2B. Neisseria gonorrhoeae—Percentage of Isolates with Elevated MICs to Azithromycin, Cefixime, and CeftriaxoneAbbreviations: GISP = Gonococcal Isolate Surveillance Project; MICs = minimum inhibitory concentrations;
Elevated MICS = Azithromycin ≥2.0 µg/mL; Cefixime ≥0.25 µg/mL; Ceftriaxone ≥0.125 µg/mLSource: Centers for Disease Control and Prevention. Gonococcal Isolate Surveillance Project (GISP) Profiles, 2022. Figure 2C. Prevalence of Tetracycline, Penicillin, or Fluoroquinolone Resistance* or Elevated Cefixime, Ceftriaxone, or Azithromycin MICs^, by YearAbbreviations: MICs = minimum inhibitory concentrations
Figure 2C. Prevalence of Tetracycline, Penicillin, or Fluoroquinolone Resistance* or Elevated Cefixime, Ceftriaxone, or Azithromycin MICs^, by YearAbbreviations: MICs = minimum inhibitory concentrations
*Resistance: Ciprofloxacin = MIC ≥ 1.0 µg/mL; Penicillin = MIC ≥2.0 µg/mL or Beta-lactamase positive; Tetracycline = MIC ≥2.0 µg/mL
^Elevated MICS = *Elevated MICs: Azithromycin: MIC ≥ 1.0 µg/mL (2000–2004); ≥ 2.0 µg/mL (2005–2022); Ceftriaxone: MIC ≥ 0.125 µg/mL; Cefixime: MIC ≥ 0.25 µg/mLSource: Centers for Disease Control and Prevention. Gonococcal Isolate Surveillance Project (GISP) Profiles, 2022. Figure 2D. Neisseria gonorrhoeae—Distribution of Gentamicin MICs by Year, 2018–2022Abbreviations: MICs = minimum inhibitory concentrations
Figure 2D. Neisseria gonorrhoeae—Distribution of Gentamicin MICs by Year, 2018–2022Abbreviations: MICs = minimum inhibitory concentrations
Note: Beginning in 2018, the antibiotic susceptibility testing range for gentamicin was expanded from MICs of 1 µg/mL–32 µg/mL in previous years to 0.25 µg/mL–64 µg/mL.Source: Centers for Disease Control and Prevention. Gonococcal Isolate Surveillance Project (GISP) Profiles, 2022. Figure 3 (Image Series). Neisseria gonorrhoeae DiplococciTransmission electron micrograph (TEM) showing Neisseria gonorrhoeae undergoing binary fission.Source: Centers for Disease Control and Prevention Public Health Image Library (Dr. Wiesner, 1972).
Figure 3 (Image Series). Neisseria gonorrhoeae DiplococciTransmission electron micrograph (TEM) showing Neisseria gonorrhoeae undergoing binary fission.Source: Centers for Disease Control and Prevention Public Health Image Library (Dr. Wiesner, 1972). Figure 3B. Neisseria gonorrhoeaeThis illustration shows a three-dimensional computer-generated image of Neisseria gonorrhoeae diplococci. The illustration is an artistic recreation based on scanning electron microscopic (SEM) imagery. Note the hair-like appendages extending from the surface membrane; these are type IV pili that promote motility and improve surface adherence.Source: Centers for Disease Control and Prevention Public Health Image Library (Medical Illustration—James Archer, 2013).
Figure 3B. Neisseria gonorrhoeaeThis illustration shows a three-dimensional computer-generated image of Neisseria gonorrhoeae diplococci. The illustration is an artistic recreation based on scanning electron microscopic (SEM) imagery. Note the hair-like appendages extending from the surface membrane; these are type IV pili that promote motility and improve surface adherence.Source: Centers for Disease Control and Prevention Public Health Image Library (Medical Illustration—James Archer, 2013). Figure 4. Purulent Urethral Discharge with Gonococcal InfectionPhotograph from Negusse Ocbamichael, PA; Public Health—Seattle & King County Sexual Health Clinic
Figure 4. Purulent Urethral Discharge with Gonococcal InfectionPhotograph from Negusse Ocbamichael, PA; Public Health—Seattle & King County Sexual Health Clinic Figure 5. CervicitisThis illustration of a woman with cervicitis shows a light purulent discharge from the cervical os seen with a speculum examination (on left) and in close up (right).Illustration by Jared Travnicek, Cognition Studio
Figure 5. CervicitisThis illustration of a woman with cervicitis shows a light purulent discharge from the cervical os seen with a speculum examination (on left) and in close up (right).Illustration by Jared Travnicek, Cognition Studio Figure 6 (Image Series). Bartholin Glands, Bartholin Cyst, and Bartholin AbscessThe Bartholin glans are located on each side of the vaginal introitus, at approximately the 4 and 8 o'clock positions. Each gland is normally oval-shaped and less than 1 cm in diameter.Illustration credit: Cognition Studio, Inc. and David H. Spach, MD
Figure 6 (Image Series). Bartholin Glands, Bartholin Cyst, and Bartholin AbscessThe Bartholin glans are located on each side of the vaginal introitus, at approximately the 4 and 8 o'clock positions. Each gland is normally oval-shaped and less than 1 cm in diameter.Illustration credit: Cognition Studio, Inc. and David H. Spach, MD Figure 6B. Bartholin CystThis illustration shows a Bartholin cyst involving the patient’s right Bartholin gland. A Bartholin cyst, is a fluid-filled sac on the labial or vaginal wall; this cyst forms from the obstruction of the Bartholin's gland.Illustration credit: Cognition Studio, Inc. and David H. Spach, MD
Figure 6B. Bartholin CystThis illustration shows a Bartholin cyst involving the patient’s right Bartholin gland. A Bartholin cyst, is a fluid-filled sac on the labial or vaginal wall; this cyst forms from the obstruction of the Bartholin's gland.Illustration credit: Cognition Studio, Inc. and David H. Spach, MD Figure 6C. Bartholin AbscessThis illustration shows Bartholin abscess involving the patient’s right Bartholin gland. A Bartholin cyst can become infected and develop into a Bartholin abscess that is tense, painful, and filled with pus.Illustration credit: Cognition Studio, Inc. and David H. Spach, MD
Figure 6C. Bartholin AbscessThis illustration shows Bartholin abscess involving the patient’s right Bartholin gland. A Bartholin cyst can become infected and develop into a Bartholin abscess that is tense, painful, and filled with pus.Illustration credit: Cognition Studio, Inc. and David H. Spach, MD Figure 7. Gonococcal ConjunctivitisThis photograph illustrates a severe case of gonococcal conjunctivitis. Note the purulent material on the upper and lower lids.Source: Centers for Disease Control and Prevention Public Health Image Library. CDC, 1977.
Figure 7. Gonococcal ConjunctivitisThis photograph illustrates a severe case of gonococcal conjunctivitis. Note the purulent material on the upper and lower lids.Source: Centers for Disease Control and Prevention Public Health Image Library. CDC, 1977. Figure 8 (Image Series). Disseminated Gonococcal InfectionSource: Centers for Disease Control and Prevention Public Health Image Library (Emory, Tom Sellers, 1963).
Figure 8 (Image Series). Disseminated Gonococcal InfectionSource: Centers for Disease Control and Prevention Public Health Image Library (Emory, Tom Sellers, 1963).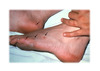 Figure 8B. Disseminated Gonococcal Infection with Skin LesionsThis patient had disseminated gonococcal infection including multiple cutaneous lesions on the feet (black arrows).Source: Centers for Disease Control and Prevention Public Health Image Library (J. Pledger and Dr. S. E. Thompson, VDCD, 1979).
Figure 8B. Disseminated Gonococcal Infection with Skin LesionsThis patient had disseminated gonococcal infection including multiple cutaneous lesions on the feet (black arrows).Source: Centers for Disease Control and Prevention Public Health Image Library (J. Pledger and Dr. S. E. Thompson, VDCD, 1979). Figure 9. Urethral Swab Gram Stain in a Patient with GonorrheaThis Gram stain of a smear of a urethral discharge in a man diagnosed with acute gonococcal urethritis.Source: Centers for Disease Control and Prevention Public Health Image Library (Joe Miller, 1979).
Figure 9. Urethral Swab Gram Stain in a Patient with GonorrheaThis Gram stain of a smear of a urethral discharge in a man diagnosed with acute gonococcal urethritis.Source: Centers for Disease Control and Prevention Public Health Image Library (Joe Miller, 1979).Tables
Table 1. 2021 STI Treatment Guidelines: Gonococcal InfectionsTreatment of Uncomplicated Gonococcal Infection of the Cervix, Urethra, or RectumRecommended Regimen if Chlamydial Infection ExcludedCeftriaxone500 mg* IM in a single dose for persons weighing <150 kgCeftriaxone
Tradename:RocephinNote: *For persons weighing ≥150 kg, ceftriaxone 1 g IM should be administered.Recommended Regimen if Chlamydial Infection Has Not Been ExcludedCeftriaxone500 mg* IM in a single dose for persons weighing <150 kgCeftriaxone
Tradename:RocephinDoxycycline100 mg orally twice daily for 7 days+Doxycycline
Tradename:Doryx, VibramycinDuring pregnancy, oral azithromycin 1 gram in a single dose is recommended to treat chlamydia.Note: *For persons weighing ≥150 kg, ceftriaxone 1 g IM should be administered.Alternative Regimen if Ceftriaxone is Not AvailableGentamicin240 mg IM in a single doseGentamicin
Tradename:GaramycinAzithromycin2 g orally in a single dose+Azithromycin
Tradename:ZithromaxAlternative Regimen if Ceftriaxone is Not AvailableCefixime800 mg orally in a single doseCefixime
Tradename:SupraxNote: If treating with cefixime, and chlamydial infection has not been excluded, providers should treat for chlamydia with doxycycline 100 mg orally twice daily for 7 days. During pregnancy, oral azithromycin 1 g in a single dose is recommended to treat chlamydia.Source: Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. Gonococcal infections. MMWR Recomm Rep. 2021;70(No. RR-4):1-187. [2021 STI Treatment Guidelines]Table 2. 2021 STI Treatment Guidelines: Gonococcal InfectionsTreatment of Uncomplicated Gonococcal Infection of the PharynxRecommended RegimenCeftriaxone500 mg* IM in a single dose for persons weighing <150 kgCeftriaxone
Tradename:RocephinIf chlamydial infection is identified when pharyngeal gonorrhea testing is performed, treat with doxycycline 100 mg orally 2 times a day for 7 days; women who are pregnant should receive azithromycin 1 g orally in a single dose (instead of doxycycline).Note: *For persons weighing ≥150 kg, ceftriaxone 1 g IM should be administered.No reliable alternative treatments are available for pharyngeal gonorrhea. For persons with a history of a beta-lactam allergy, a thorough assessment of the reaction is recommended. For persons with an anaphylactic or other severe reaction (e.g., Stevens Johnson syndrome) to ceftriaxone, consult an infectious disease specialist for an alternative treatment recommendation.Source: Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. Gonococcal infections. MMWR Recomm Rep. 2021;70(No. RR-4):1-187. [2021 STI Treatment Guidelines]Table 3. 2021 STI Treatment Guidelines: Gonococcal InfectionsTreatment of Gonococcal Conjunctivitis Among Adolescents and AdultsRecommended RegimenCeftriaxone1 g IM in a single doseCeftriaxone
Tradename:RocephinProviders should consider one-time lavage of the infected eye with saline solution.Source: Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. Gonococcal infections. MMWR Recomm Rep. 2021;70(No. RR-4):1-187. [2021 STI Treatment Guidelines]Table 4. 2021 STI Treatment Guidelines: Gonococcal InfectionsTreatment of Disseminated Gonococcal Infection (DGI): Arthritis and Arthritis-Dermatitis SyndromeRecommended RegimenCeftriaxone1 g IM or IV every 24 hoursCeftriaxone
Tradename:RocephinNote: If chlamydial infection has not been excluded, providers should treat for chlamydia with doxycycline 100 mg orally 2 times/day for 7 days.Alternative RegimensCefotaxime1 gram IV every 8 hoursCefotaxime
Tradename:ClaforanNote: If chlamydial infection has not been excluded, providers should treat for chlamydia with doxycycline 100 mg orally 2 times/day for 7 days.Alternative RegimensCeftizoxime1 g IV every 8 hoursCeftizoxime
Tradename:CefizoxNote: If chlamydial infection has not been excluded, providers should treat for chlamydia with doxycycline 100 mg orally 2 times/day for 7 days.When treating for the arthritis-dermatitis syndrome, the provider can switch to an oral agent guided by antimicrobial susceptibility testing 24–48 hours after substantial clinical improvement, for a total treatment course of at least 7 days.Source: Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. Gonococcal infections. MMWR Recomm Rep. 2021;70(No. RR-4):1-187. [2021 STI Treatment Guidelines]Table 5. 2021 STI Treatment Guidelines: Gonococcal InfectionsTreatment of Disseminated Gonococcal Infection: Meningitis and EndocarditisRecommended RegimenCeftriaxone1–2 g IV every 24 hoursCeftriaxone
Tradename:RocephinNote: If chlamydial infection has not been excluded, providers should treat for chlamydia with doxycycline 100 mg orally 2 times a day for 7 days.No recent studies have been published regarding treatment of DGI involving the CNS or cardiovascular system. The duration of treatment for DGI in these situations has not been systematically studied and should be determined in consultation with an infectious disease specialist. Treatment for DGI should be guided by the results of antimicrobial susceptibility testing. Length of treatment should be determined based on clinical presentation. Therapy for meningitis should be continued with recommended parenteral therapy for 10–14 days. Parenteral antimicrobial therapy for endocarditis should be administered for >4 weeks.Source: Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. Gonococcal infections. MMWR Recomm Rep. 2021;70(No. RR-4):1-187. [2021 STI Treatment Guidelines]Share by e-mail
Check
-On-
Learning
QuestionsThe Check-on-Learning Questions are short and topic related. They are meant to help you stay on track throughout each lesson and check your understanding of key concepts.You must be signed in to customize your interaction with these questions.
Since you've received 80% or better on this quiz, you may claim continuing education credit.
You seem to have a popup blocker enabled. If you want to skip this dialog please Always allow popup windows for the online course.
Current Version: nstdc-monorepo-0406a550-2025-04-04-232359Become a New UserAccount Registration Benefits:
- Track your progress on the 9 lessons
- Earn free CNE/CME/CE
- Earn Certificates of Completion
- Access to other free IDEA curricula
Create a free account to get started
- 0%Lesson 2




















