Pelvic inflammatory disease (PID) is a clinical syndrome characterized by infection and inflammation of the upper female genital tract. This process results from the ascending spread of microorganisms in the vagina or cervix to the structures of the upper female genital tract, with potential infection and inflammation of the endometrium, fallopian tubes, pelvic peritoneum, and, in some instances, formation of tubo-ovarian abscess.[1,2] In recent years, the range of microorganisms believed to play a major role in this process has expanded.[3] Typically, PID is classified as either acute (less than or equal to 30 days duration), subclinical (asymptomatic disease of unknown duration), or chronic (greater than 30 days duration) (Figure 1).[1] The diagnosis of PID may be challenging as women with PID often experience subtle clinical manifestations, and precise diagnostic criteria are lacking.[4] Women who experience PID may have adverse reproductive sequelae, including infertility, ectopic pregnancy, and chronic pelvic pain.[5,6] Effective parenteral and oral treatments are available for PID that provide short-term clinical benefit and reduce the risk of developing long-term complications.[1,7] When considering the severe potential long-term consequences associated with PID, it is extremely important for clinicians to diagnose PID and promptly provide appropriate and effective antimicrobial therapy.
- Module 2 Overview
Self-Study Lessons - 0%Lesson 1
Chlamydial InfectionsActivities- 0%Lesson 2
Gonococcal InfectionsActivities- 0%Lesson 3
SyphilisActivities- 0%Lesson 4
Syphilis During Pregnancy and Congenital SyphilisActivities- 0%Lesson 5
Genital HerpesActivities- 0%Lesson 6
Human Papillomavirus InfectionActivities- 0%Lesson 7
Pelvic Inflammatory DiseaseActivities- 0%Lesson 8
VaginitisActivities- 0%Lesson 9
MpoxActivities- 0%Lesson 10
Mycoplasma genitaliumActivitiesLesson 7. Pelvic Inflammatory Disease
PDF ShareLast Updated: March 20th, 2025Authors:David H. Spach, MD,David H. Spach, MD
Editor-in-Chief
Professor of Medicine
Division of Allergy and Infectious Diseases
University of WashingtonDisclosures: NoneKaren A. Wendel, MD,Karen A. Wendel, MD
Director, HIV/STI Prevention and Control
Denver Public Health
Medical Director, Denver Prevention Training CenterDisclosures: NoneKatherine Hsu, MD, MPH, FAAPKatherine Hsu, MD, MPH, FAAP
Medical Director, Division of STD Prevention & HIV/AIDS Surveillance
Director, Sylvie Ratelle STD/HIV Prevention Training Center
Massachusetts Department of Public Health
Professor of Pediatrics
Section of Pediatric Infectious Diseases
Boston University Medical CenterDisclosures: NoneLearning Objective Performance Indicators
- Summarize the pathogenesis and common organisms that cause pelvic inflammatory disease
- Recognize clinical manifestations of pelvic inflammatory disease
- Use established clinical criteria to diagnose pelvic inflammatory disease
- Treat pelvic inflammatory disease with recommended antimicrobial regimens
- Provide counseling messages for possible long-term complications of pelvic inflammatory disease
Table of Contents- Pelvic Inflammatory Disease
- Introduction
- Epidemiology
- Microbiology and Pathogenesis
- Clinical Manifestations
- Diagnosis
- Treatment
- General Considerations for Treatment
- Hospital Admission Criteria with Acute PID
- Parenteral Treatment
- Intramuscular/Oral Treatment
- Regimens In Persons with Cephalosporin Allergy
- Management of PID in Adolescents and Young Adults
- Management of PID in Women with HIV
- Management of Tubo-Ovarian Abscess
- Recent Placement of Intrauterine Device
- Follow-Up
- Management of Sex Partners
- Counseling and Education
- Summary Points
- Check-On-Learning
- Citations
- Additional References
- Figures
- Tables
Introduction
Epidemiology
Prevalence
It is difficult to accurately estimate the incidence and prevalence of PID in the United States as there is no single diagnostic test for PID, and it is not a reportable disease. In a comprehensive review of two sentinel data sources (National Health and Nutrition Examination Survey [NHANES] and the National Survey of Family Growth [NSFG]), investigators summarized the burden of and trends in PID among reproductive-aged women (18 to 44 years of age) in the United States and estimated a 4% self-reported history of PID and estimated 2 million or more reproductive-aged women in the United States having a diagnosis of PID in their lifetime.[8] For women with a previously diagnosed sexually transmitted infection (STI), the lifetime prevalence of PID has been estimated as even higher, at approximately 10%.[9]
Trends in PID
Available data from national insurance claims, visits to office-based physicians, and emergency departments point to an overall trend of decline in the incidence of PID in the United States since the year 2000.[8,10,11] In the NHANES and NSFG review, data from the NSFG component of this study showed a decline in the prevalence of PID from 4.9% in 2006 to 3.6% in 2017, representing an overall decrease of 26.5% during this time.[8] The PID rates have been highest in the South and in non-Hispanic Black women.[8] Factors such as access to testing and care may play a role in differences in PID prevalence. Though precise reasons for the overall decline in PID since 2000 are not known, some of the decline has been attributed to increased screening for Chlamydia trachomatis and Neisseria gonorrhoeae in young women.[12,13,14,15] In addition, there were likely some additional declines during the COVID-19 pandemic period.[16]
Factors that Impact Risk for PID
Epidemiologic studies have revealed numerous factors associated with PID, and many of these factors overlap with those known to be associated with acquisition of infections that cause PID. Multiple sex partners, age younger than 20 years, and current or prior infection with gonorrhea or chlamydia have consistently been demonstrated as significant factors associated with women developing PID.[17] Other possible risk factors include a prior history of PID, male partners with gonorrhea or chlamydia, current douching, recent insertion of an intrauterine device (IUD), bacterial vaginosis, and oral contraceptive use.[18,19] The following provides greater detail on major factors associated with risk of developing PID:
- Age and Age of Sexual Debut: Several studies have identified age less than 20 years as a major risk factor for the development of PID.[17,20] The increased risk of PID in younger women correlates with the high rates of chlamydia and gonorrhea in female adolescents and young adult women. In addition, cervical ectopy—the composition of the cervical epithelium that is often present in adolescents—allows for more efficient access of infectious pathogens to the vulnerable target cells. Younger age at sexual debut is also a risk factor for PID. In the NHANES 2013-2014, In the NHANES 2013-2014, a total of 1,171 sexually experienced women 18 to 44 years of age were interviewed regarding a lifetime diagnosis of PID, and the lifetime PID prevalence in sexually experienced women 18 to 44 years of age was highest in those with the earliest sexual debut (Figure 2).[9]
- Number of Sex Partners: Several studies have shown a correlation between a greater number of sex partners and the risk of PID. In the NHANES 2013-2014, a total of 1,171 sexually experienced women 18 to 44 years of age were interviewed regarding a lifetime diagnosis of PID, and the lifetime PID prevalence was approximately three times greater in women with 10 or more lifetime vaginal sex partners than in women with one partner (Figure 3).[9]
- Condom Use: In a study of 684 sexually active women with PID who were followed for a mean duration of 35 weeks, consistent condom use (about 60% of encounters) reduced the risk of recurrent PID, chronic pelvic pain, and infertility by 30 to 60%.[21]
- Screening for Chlamydia: Several studies have shown that screening young, sexually active women for cervical chlamydial infection, with treatment of chlamydia for those who test positive, can substantially reduce the incidence of PID.[22,23]
- History of PID: A prior history of PID increases the risk of developing recurrent infection.[24] The damage that occurs to the fallopian tube mucosa during an episode of PID makes women more susceptible to recurrent infection. Likewise, a history of a gonococcal or chlamydial infection increases the likelihood of recurrent disease, which then increases the risk for PID.
- Vaginal Douching: Douching is thought to increase the risk for PID because it contributes to vaginal flora changes, epithelial damage, and disruption of the cervical mucous barrier, all of which can increase the likelihood of developing PID.[18,19] The relationship between douching has been called into question in more recent studies, and the recommendation for or against vaginal douching is currently subject to debate.[25]
- Bacterial Vaginosis: The relationship of bacterial vaginosis to PID is similarly unclear. Although the anaerobic bacteria associated with bacterial vaginosis have also been detected in the upper genital tract in association with PID, epidemiologic analyses have not consistently shown a clear association between bacterial vaginosis and the development of PID.[25]
- Recent Placement of an Intrauterine Device: The insertion of an intrauterine device (IUD) has been shown to increase the risk of PID approximately 6-fold within the first 21 days of placement, but after 21 days, the risk returns to baseline.[26,27,28] The 2024 United States Medical Eligibility Criteria for Contraceptive Use (US MEC) recommended that purulent cervicitis or current N. gonorrhoeae or C. trachomatis infection are contraindications to IUD insertion.[29] This recommendation has been controversial as several studies have noted rates of PID among new IUD users that were 1% or lower, regardless of whether they tested positive at the time of the IUD insertion for N. gonorrhoeae and/or C. trachomatis.[30,31,32]
Microbiology and Pathogenesis
Organisms Associated with PID
The organisms associated with PID depend on whether the PID is acute (duration of 30 days or less) or chronic (duration of 30 days or more) (Table 1).[1] Most cases of acute PID are polymicrobial, but in many cases, no pathogen is identified.[3,20,33] The most common pathogens identified with acute PID are N. gonorrhoeae and C. trachomatis; earlier studies identified one (or both) of these pathogens in approximately 50% of cases of PID.[34,35,36] More recently, the spectrum of organisms identified that cause PID has expanded, and the proportion of cases caused by N. gonorrhoeae or C. trachomatis has decreased to less than 50%.[3,37,38] Other microbes that likely cause PID (and are considered to be rising in PID incidence) include Mycoplasma genitalium, Trichomonas vaginalis, bacterial vaginosis-associated bacteria (multiple anaerobic organisms), and, less frequently, bacteria associated with the gastrointestinal tract (Bacteroides species, Escherichia coli).[2,3,39,40,41] Although there is a lack of prospective data demonstrating causality, a recent systematic review and meta-analysis reported that M. genitalium was associated with a 67% increase in odds of PID and was detected in 10% of patients with PID.[42] Chronic PID is more often caused by Mycobacterial tuberculosis and Actinomyces species.[1]
Pathway of Ascendant Infection
The intermittent ascension of microorganisms from the lower genitourinary tract into the endometrial cavity and fallopian tubes likely occurs as a normal physiological phenomenon (Figure 4). Whether these organisms cause PID depends on the organism’s viability, number, pathogenicity, and immune defense mechanisms of the host. Host immunogenetic variations have been invoked as contributing factors to the development of PID; bacterial factors do not fully explain the differences observed in clinical manifestations or the complications.[43,44]
Pathogenesis of Reproductive Damage
With acute PID, the ascending organisms trigger an inflammatory response that involves the endometrium, fallopian tubes, and/or the pelvic peritoneum.[1,5] The normal fallopian tube tissue has millions of tiny hair-like cilia that beat in waves that assist in transporting the egg through the tube to the uterine cavity. As a result of inflammation and tissue destruction, the fallopian tube may have a loss of cilia, leading to dysregulation of egg transport and an increased risk of ectopic pregnancy (Figure 5).[1] The damage and scarring caused by PID may lead to the described sequelae of infertility, ectopic pregnancy, and chronic pelvic pain.[1,45,46] This can occur even in women who do not report a history of PID symptoms and is often referred to as subclinical PID.[6,47]
Clinical Manifestations
Signs and Symptoms
Women with acute or subacute PID present with a wide array of clinical manifestations that range from asymptomatic or subclinical infection to severe and debilitating symptoms.[1,6,48] Women with acute PID may experience subtle, nonspecific symptoms such as dyspareunia, dysuria, or gastrointestinal symptoms, which they may not attribute to pelvic infection.[49] In this situation, many women do not seek medical care, or they present with these nonspecific findings that may make it challenging for the medical provider to diagnose PID.[6,47] When mild to moderate symptoms of PID do occur, women may describe lower abdominal or pelvic pain that is accentuated by coitus. Other common symptoms include cramping, dysuria, urinary frequency, vaginal discharge, and intermittent or postcoital cervical bleeding. Systemic signs, such as fever, chills, nausea, and vomiting, are often absent in mild to moderate cases. Examination of the cervix may demonstrate discharge, friability, or motion tenderness. In addition, lower abdominal palpation may show uterine or adenexal pain and tenderness.[1,5,7] In severe PID, women may appear very ill and may have additional findings of fever, chills, purulent vaginal discharge, nausea, and vomiting. If PID has been complicated by the development of a tubo-ovarian abscess, the examination may be notable for an adnexal mass or fullness.
Alternative and Overlapping Diagnoses
Since many women with PID present with nonspecific clinical manifestations, it is important to consider other diseases that may overlap and appear similar to PID. The main considerations for alternative and overlapping diagnoses are appendicitis, ectopic pregnancy, endometriosis, endometritis, ovarian cyst (with or without rupture), nephrolithiasis, and urinary tract infection.[5] A thorough clinical evaluation with appropriate laboratory tests can usually differentiate these processes from PID.
Acute and Subacute Complications Associated with PID
Women with acute PID can develop a range of inflammatory complications, including local tissue damage, fallopian tube swelling, development of adhesions, and tubal occlusion (Figure 6).[49,50]
- Fitz-Hugh-Curtis Syndrome: The inflammatory process with PID can extend to the liver capsule, a process commonly referred to as perihepatitis or Fitz-Hugh-Curtis syndrome (Figure 7).[5,51] The hepatic capsular inflammation can be associated with adhesions between the liver capsule and the anterior abdominal wall. An estimated 1 to 30% of women with PID develop this complication.[52] Women with Fitz-Hugh-Curtis syndrome typically present with right upper quadrant pain that is usually accentuated with movement or inspiration. Abdominal ultrasonography or contrast computed tomography can support the diagnosis with a finding of increased perihepatic enhancement, usually of the right lobe of the liver; imaging can also help to rule out other causes of right upper quadrant pain.[51] Direct visualization with laparoscopy can confirm the diagnosis by showing characteristic violin string-like adhesions between the surface of the liver and the anterior abdominal wall.[52] Management of Fitz-Hugh-Curtis syndrome requires treatment of the underlying cause of PID. Surgical management would be considered in the setting of symptomatic adhesions and/or the presence of an abscess.[53]
- Tubo-ovarian Abscess: Tubo-ovarian abscess is an inflammatory mass involving a fallopian tube, ovary, or both; the mass is characterized by the presence of abundant pus (Figure 8).[50] It is a known complication of PID and has been reported in approximately 30% of women hospitalized with PID.[54] The most common clinical manifestations associated with tubo-ovarian abscess are abdominal or pelvic pain, fever, vaginal discharge, nausea, and abnormal vaginal bleeding; approximately 25% will have a normal white blood cell count.[54,55] The reproductive outcome for women with tubo-ovarian abscess depends on whether surgical intervention is required and whether intraabdominal rupture occurs. If intraabdominal rupture is suspected and women are treated with fertility-preserving, conservative surgery, the reported subsequent pregnancy rate is 25%. For women without rupture who are treated with medical management alone, reported pregnancy rates vary between 4% and 15%.[50] One retrospective cohort study of women hospitalized with PID or tubo-ovarian abscess found that 25.5% of women subsequently met the criteria of infertility, 16.0% had recurrent PID, and 13.8% reported chronic pelvic pain.[56]
Chronic Sequelae Associated with PID
The sequelae of PID, including ectopic pregnancy, infertility, or chronic pelvic pain, may occur after a single episode of symptomatic PID. In addition, available data suggest women with subclinical PID can develop long-term sequelae, including infertility.[6,47] The development of “silent PID” poses a major diagnostic and treatment challenge.[7] Appropriate therapy has been shown to significantly decrease the rate of long-term sequelae.[57] In contrast, delays in therapy for PID or repeated episodes of PID significantly increase the risk of developing long-term complications.[35,58,59]
- Ectopic Pregnancy: In one PID treatment study, among women with documented salpingitis, the subsequent risk of ectopic pregnancy was 9%.[59]
- Chronic Pelvic Pain: Following treatment of PID, chronic pelvic pain is common. One large PID treatment study reported that 29% of women had chronic pelvic pain (pain reported at two or more consecutive visits 3 to 4 months apart during a period of 2 to 5 years) after receiving treatment for PID.[35]
- Tubal Infertility: Infertility is typically defined as a woman’s inability to conceive after 1 year of attempting to become pregnant. In developed countries worldwide, tubal problems comprise approximately 20% of the causes of infertility, and PID is the most common cause of tubal factor infertility.[60,61,62] In several PID treatment studies that enrolled women with mild-to-moderate PID, 16-18% of women reported infertility.[35,59] Tubal infertility increases with multiple episodes of PID or more severe cases of PID (Figure 9).[59]
Diagnosis
Due to the difficulty of diagnosis and the potential for damage to the reproductive health of women, health care providers should maintain a high index of suspicion for PID.[35] Acute PID is difficult to diagnose due to the wide range of clinical presentations associated with the illness. No single physical finding, image, or laboratory test can reliably make a definitive diagnosis.[63] Given the importance of prompt diagnosis and treatment of PID, the initial diagnosis is often made based on clinical findings.[7,64] Several studies suggest the positive predictive value of a clinical diagnosis for symptomatic PID, when compared with laparoscopy, is in the range of 65 to 90%.[65,66,67,68]
Recommended Initial Diagnostic Evaluation
Routine initial laboratory testing for women with possible PID should include saline microscopy of vaginal fluid, nucleic acid amplification testing (NAAT) for chlamydia and gonorrhea, urinalysis (with culture if indicated), and pregnancy test.[7] If there is a need to increase the diagnostic specificity for PID, additional initial studies should include a complete blood count, C-reactive protein, and erythrocyte sedimentation rate. In addition, testing for M. genitalium infection in the initial evaluation of PID should be considered.[7] Radiographic imaging and laparoscopy can help to confirm a clinical diagnosis of PID, and obtaining samples for culture during laparoscopy can also provide a microbiologic diagnosis. In many situations, laparoscopy may not be indicated or available. In addition to the diagnostic evaluation, all persons who possibly have PID should have syphilis and HIV testing as part of their initial evaluation.[7]
Diagnostic Criteria
The following summarizes the main criteria recommended in the 2021 STI Treatment Guidelines for making a diagnosis of PID, including criteria for initiating presumptive treatment.[7]
Criteria for Initiating Presumptive Treatment for PID
- Presumptive PID treatment should be initiated for sexually active young women and other women at risk for STIs who are experiencing pelvic or lower abdominal pain unexplained by another illness AND if they meet at least one of the following three clinical criteria on pelvic examination:
- Cervical motion tenderness, or
- Uterine tenderness, or
- Adnexal tenderness
Additional Clinical Criteria
- One or more of the following additional criteria can be used to enhance the specificity of the minimum clinical criteria to support a diagnosis of PID.
- Oral temperature greater than 38.3°C (greater than 101°F)
- Abnormal cervical mucopurulent discharge or cervical friability
- Presence of abundant numbers of white blood cells on saline microscopy of vaginal fluid
- Elevated erythrocyte sedimentation rate
- Elevated C-reactive protein
- Laboratory documentation of cervical infection with N. gonorrhoeae or C. trachomatis
Specific Diagnostic Criteria
- In some women with suspected PID, more extensive evaluation, such as radiographic imaging, biopsy, or laparoscopy, is warranted. Endometrial biopsy is indicated in women who are undergoing laparoscopy but do not have visual evidence of salpingitis; in this situation, endometritis may be the only objective sign of PID. The following summarizes specific criteria used to make a diagnosis of PID if biopsy, radiographic, or laparoscopic procedures are performed.
- Endometrial biopsy with histopathologic evidence of endometritis, or
- Transvaginal sonography or magnetic resonance imaging techniques showing thickened, fluid-filled tubes with or without free pelvic fluid or tubo-ovarian complex, or Doppler studies suggesting pelvic infection (e.g., tubal hyperemia), or
- Laparoscopic findings are consistent with PID.[7]
- If a diagnostic pathogen has not been identified and the response to initial treatment is not certain, testing for M. genitalium should be performed, unless this has already been done. The rationale for this recommendation is that empiric PID treatments do not adequately treat M. genitalium infection.
Reporting Requirements
Although there is no specific reporting requirement for PID, all states have laws and regulations that require clinicians, laboratories, or both, to report women with PID who have a positive test for gonorrhea or chlamydia to public health authorities.
Treatment
General Considerations for Treatment
Clinicians should have a low threshold for diagnosing and promptly treating PID in sexually active women with pelvic or lower abdominal pain.[7] Treatment should not be withheld while waiting for STI testing results.[5] Timely administration of antimicrobial therapy improves outcomes and reduces the risk of long-term adverse sequelae. In a case-control study that included 443 women with PID, delayed care (treatment 3 or more days after onset of abdominal pain) was associated with a 3-fold increase in infertility or ectopic pregnancy.[58] The empiric treatment regimens should provide broad-spectrum coverage of the likely causative pathogens, most notably N. gonorrhoeae, C. trachomatis, and anaerobic organisms. A negative endocervical, vaginal, or urine screening for gonorrhea and chlamydia does not rule out upper genital tract infection with these pathogens. The importance of empirically treating M. genitalium is unknown, and most regimens do not provide reliable treatment for M. genitalium; if treatment for M. genitalium is indicated, moxifloxacin is the treatment of choice.[7] It is important to note when using moxifloxacin to treat PID, the duration of therapy should be 14 days.[7] Multiple parenteral, oral, and parenteral-oral combination antimicrobial regimens have been effective in achieving clinical and microbiologic cure in clinical trials.[69,70,71,72,73,74] There are sufficient data to support treatment of PID with oral regimens, parenteral antimicrobials, or a combination of both, depending on the severity of the clinical illness.[1,7,37,75]
Hospital Admission Criteria with Acute PID
The decision of whether to hospitalize for more intense monitoring and treatment can be challenging. This decision should be made based on the medical provider’s clinical judgment in conjunction with an assessment for criteria that indicate a need for inpatient monitoring and care. The following are suggested criteria for the hospitalization of women with PID.[7]
- Inability to exclude surgical emergencies (e.g., appendicitis, ectopic pregnancy)
- Tubo-ovarian abscess
- Pregnancy
- Severe illness, nausea and vomiting, or temperature greater than 38.5°C (101°F)
- Inability to follow or tolerate an outpatient oral regimen
- Nonresponse to oral therapy, as defined by failure to respond clinically to outpatient antimicrobial therapy within 48 to 72 hours
Parenteral Treatment
Multiple randomized trials have demonstrated the efficacy of parenteral regimens for the treatment of acute PID.[37,72,74,75,76] For initial parenteral therapy for PID, there are three recommended and two alternative regimens.[7] The three recommended parenteral regimens consist of a cephalosporin plus doxycycline, with or without metronidazole, depending on the cephalosporin (see Table 2 below for dosing and duration):
- Ceftriaxone plus doxycycline plus metronidazole, or
- Cefotetan plus doxycycline, or
- Cefoxitin plus doxycycline
The ceftriaxone plus doxycycline regimen requires the addition of metronidazole to provide adequate activity against anaerobic organisms. With the other two recommended regimens (cefotetan plus doxycycline or cefoxitin plus doxycycline), the addition of metronidazole is not necessary because cefotetan and cefoxitin both have strong anti-anaerobic activity. If the person receiving treatment can reliably tolerate oral administration of medications, administering doxycycline orally is preferred over intravenously to avoid pain that can be associated with doxycycline infusions. Within 24 to 48 hours of clinical improvement, therapy can be transitioned from parenteral to oral therapy to complete 14 days of antimicrobial treatment.[7] For persons with a severe cephalosporin allergy, the alternative regimen of clindamycin plus gentamicin is an option for parenteral therapy.
Table 2. 2021 STI Treatment Guidelines: Pelvic Inflammatory Disease (PID)Parenteral RegimensClinical experience should guide decisions regarding transition to oral therapy, which usually can be initiated within 24–48 hours of clinical improvement. The oral regimen should be based on the initial parenteral regimen, as outlined below. In women with tubo-ovarian abscesses, at least 24 hours of inpatient observation is recommended.Recommended RegimensCeftriaxone1 g IV every 24 hoursCeftriaxone
Tradename:RocephinDoxycycline100 mg orally or IV every 12 hours*+Doxycycline
Tradename:Doryx, VibramycinMetronidazole500 mg orally or IV every 12 hours^+Metronidazole
Tradename:Flagyl*Because of the pain associated with IV infusion, doxycycline should be administered orally when possible. Oral and IV administration of doxycycline provide similar bioavailability.^Oral metronidazole is well absorbed and can be considered instead of IV for women without severe illness or tubo-ovarian abscess when possible.Note: Within 24 to 48 hours of clinical improvement, therapy can be transitioned to oral therapy with doxycycline 100 mg twice daily plus metronidazole 500 mg twice daily to complete 14 days of antimicrobial therapy.Recommended RegimensCefotetan2 g IV every 12 hoursCefotetan
Tradename:CefotanDoxycycline100 mg orally or IV every 12 hours*+Doxycycline
Tradename:Doryx, Vibramycin*Because of the pain associated with intravenous infusion, doxycycline should be administered orally when possible. Oral and IV administration of doxycycline provide similar bioavailability.Note: Within 24 to 48 hours of clinical improvement, therapy can be transitioned to oral therapy with doxycycline 100 mg twice daily plus metronidazole 500 mg twice daily to complete 14 days of antimicrobial therapy.Recommended RegimensCefoxitin2 g IV every 6 hoursCefoxitin
Tradename:MefoxinDoxycycline100 mg orally or IV every 12 hours+Doxycycline
Tradename:Doryx, VibramycinBecause of the pain associated with intravenous infusion, doxycycline should be administered orally when possible. Oral and IV administration of doxycycline provide similar bioavailability.Note: Within 24 to 48 hours of clinical improvement, therapy can be transitioned to oral therapy with doxycycline 100 mg twice daily plus metronidazole 500 mg twice daily to complete 14 days of antimicrobial therapy.Alternative RegimensAmpicillin-Sulbactam3 g IV every 6 hoursAmpicillin-Sulbactam
Tradename:UnasynDoxycycline100 mg orally or IV every 12 hours*+Doxycycline
Tradename:Doryx, Vibramycin*Because of the pain associated with intravenous infusion, doxycycline should be administered orally when possible. Oral and IV administration of doxycycline provide similar bioavailability.Note: Within 24 to 48 hours of clinical improvement, therapy can be transitioned to oral therapy with doxycycline 100 mg twice daily plus metronidazole 500 mg twice daily to complete 14 days of antimicrobial therapy.Alternative RegimensClindamycin900 mg IV every 8 hoursClindamycin
Tradename:CleocinGentamicinloading dose IV or IM (2 mg/kg body weight), followed by a maintenance dose (1.5 mg/kg body weight) every 8 hours; single daily dosing (3–5 mg/kg body weight) can be substituted+Gentamicin
Tradename:GaramycinNote: Within 24 to 48 hours of clinical improvement, therapy can be transitioned to oral therapy with clindamycin 450 mg four times a day or doxycycline 100 mg twice daily to complete 14 days of antimicrobial therapy.Note: if tubo-ovarian abscess is present, the regimen used when transitioning to oral therapy should consist of doxycycline plus either clindamycin 450 mg four times a day or metronidazole 500 mg twice daily to complete 14 days of antimicrobial therapy.Source: Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. Pelvic inflammatory disease (PID). MMWR Recomm Rep. 2021;70(No. RR-4):1-187. [2021 STI Treatment Guidelines]Intramuscular/Oral Treatment
Intramuscular/oral therapy can be considered for women with mild-to-moderately severe acute PID because the clinical outcomes among women treated with the recommended regimens are similar to those treated with intravenous therapy, which consists of a cephalosporin plus doxycycline, with or without metronidazole depending on the cephalosporin (see Table 3 below for dosing and duration).[35,71,76] Women receiving oral therapy for PID should have follow-up within 72 hours, at which time they should show substantial clinical improvement. If no improvement occurs by 72 hours, reevaluation should take place to confirm the diagnosis, and the oral regimen should be switched to parenteral therapy, usually in the inpatient setting.[7]
Table 3. 2021 STI Treatment Guidelines: Pelvic Inflammatory Disease (PID)Intramuscular or Oral RegimensFor women with mild-to-moderate acute PID, intramuscular (IM) or oral therapy can be considered because the clinical outcomes among women treated with these regimens are similar to those treated with IV therapy. Women who do not respond to IM/oral therapy within 72 hours should be reevaluated to confirm the diagnosis and should be administered intravenous therapy.Recommended RegimensCeftriaxone500 mg IM in a single dose*Ceftriaxone
Tradename:RocephinDoxycycline100 mg orally twice a day for 14 days+Doxycycline
Tradename:Doryx, VibramycinMetronidazole500 mg orally twice a day for 14 days+Metronidazole
Tradename:Flagyl*For persons weighing ≥150 kg, 1 gm of ceftriaxone should be administered.Recommended RegimensCefoxitin2 g IM in a single doseCefoxitin
Tradename:MefoxinProbenecid1 g orally in a single dose (given concurrently with cefoxitin)+Probenecid
Tradename:Doxycycline100 mg orally twice a day for 14 days+Doxycycline
Tradename:Doryx, VibramycinMetronidazole500 mg orally twice a day for 14 days+Metronidazole
Tradename:FlagylThe cefoxitin and probenecid should be administered concurrently.Recommended RegimensOther parenteral third-generation cephalosporin (e.g., ceftizoxime or cefotaxime)Other parenteral third-generation cephalosporin (e.g., ceftizoxime or cefotaxime)
Tradename:Doxycycline100 mg orally twice a day for 14 days+Doxycycline
Tradename:Doryx, VibramycinMetronidazole500 mg orally twice a day for 14 days+Metronidazole
Tradename:FlagylAlternative RegimensLevofloxacin500 mg orally once daily for 14 daysLevofloxacin
Tradename:LevaquinMetronidazole500 mg orally twice a day for 14 days+Metronidazole
Tradename:FlagylNote: This alternative regimen is only for consideration if the patient has cephalosporin allergy, the community prevalence and individual risk for gonorrhea are low, and follow-up is likely.Alternative RegimensMoxifloxacin400 mg orally once daily for 14 daysMoxifloxacin
Tradename:AveloxNote: This alternative regimen is only for consideration if the patient has cephalosporin allergy, the community prevalence and individual risk for gonorrhea are low, and follow-up is likely. Moxifloxacin is the preferred quinolone antimicrobial for M. genitalium infections; however, the importance of providing coverage for M. genitalium is unknown.Alternative RegimensAzithromycin500 mg IV daily for 1–2 doses, followed by 250 mg orally daily for a total of 7 daysAzithromycin
Tradename:ZithromaxMetronidazole500 mg orally three times a day for 12-14 days±Metronidazole
Tradename:FlagylNote: This alternative regimen is only for consideration if the patient has cephalosporin allergy, the community prevalence and individual risk for gonorrhea are low, and follow-up is likely.Source: Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. Pelvic inflammatory disease (PID). MMWR Recomm Rep. 2021;70(No. RR-4):1-187. [2021 STI Treatment Guidelines]Regimens In Persons with Cephalosporin Allergy
There are published data for several alternative regimens that should only be considered for use if the person diagnosed with PID has a cephalosporin allergy, the community prevalence and individual risk for gonorrhea are low, and follow-up is likely.[77,78,79,80] For women diagnosed with PID who have a cephalosporin allergy, limited data suggest the Alternative Therapy options listed in Table 3 could be used, if the PID occurs in a setting of low risk for gonorrhea.[7] In addition, if M. genitalium is diagnosed in a woman with PID, the treatment should include moxifloxacin for 14 days.
Management of PID in Adolescents and Young Adults
There are no studies that have shown adolescents and young adults with PID have better outcomes with hospitalization versus management in the outpatient setting. In addition, available data suggest that adolescents with PID have similar clinical response rates as older women when both are treated in an outpatient setting.[7] Therefore, adolescents should receive the same treatment approach as older women, taking into account whether the adolescent can adhere to outpatient management and present to care for follow-up.[7] Although the treatment approach with adolescents is usually the same as with adults, it is important to consider that adolescents may have barriers to diagnosis and care for PID, such as lack of awareness, confidentiality concerns, and difficulties accessing care.[81] The Technology Enhanced Community Health Nursing (TECH-N) model has been shown to be a feasible and acceptable intervention that can support precision PID management for adolescents and young adults.[82,83] This program provides community health nursing education, daily text medication reminders, and a follow-up visit by a community health nurse after 3 to 5 days of treatment.[82,83] The goal of this program is to provide adolescents and young adults with a cost-effective nursing approach to enhance PID care.[82,83]. A recent modeling study suggests TECH-N implementation yields better outcomes at a lower cost.[84]
Management of PID in Women with HIV
The management of PID in women with HIV is generally the same as in women without HIV. Early observational study data suggest that some women with HIV and PID have an altered immune response to an upper genital tract infection, which may contribute to a reduced response to antimicrobial therapy, longer hospital courses, greater risk of tubo-ovarian abscess, and a higher rate of required surgical intervention.[85,86,87,88] Other studies, however, have indicated that women with HIV have similar symptoms, manifestations, and treatment responses as women without HIV.[88,89,90]
Management of Tubo-Ovarian Abscess
Women with suspected or diagnosed tubo-ovarian abscess should undergo hospitalization for intensive management, including prompt receipt of intravenous antimicrobial therapy and expert consultation. A minimum of 24 hours of inpatient observation is recommended for women with a tubo-ovarian abscess.[7] The antimicrobial regimens used to treat tubo-ovarian abscess are usually consistent with recommended parenteral PID regimens.[7] Radiographic imaging can confirm the presence of a tubo-ovarian abscess, and it can be used to track response to therapy. In some women, further intervention may be needed, particularly if there are signs of rupture, evidence of hemodynamic instability, the presence of a large tubo-ovarian abscess (7 cm or greater), or there is poor response to medical therapy. The most common indications for surgery (or image-guided drainage) are failure to improve clinically or evidence of a persistent abscess on interval imaging. Notably, 85% of abscesses with a diameter of 4 to 6 cm resolve with antibiotic therapy alone, whereas only 40% of those 10 cm or larger respond.[91] An estimated 15% of women with PID and tubo-ovarian abscess will experience spontaneous rupture of the abscess, which can be life-threatening and require emergency surgery.[50,92]
Recent Placement of Intrauterine Device
For women who develop PID after a recent IUD insertion, treatment for PID can be initiated without removal of the IUD if close follow-up is arranged.[7,29,93] If, however, there is no clinical improvement after 48 to 72 hours of antimicrobial treatment, then consideration should be given to removing the IUD.[7,29]
Follow-Up
Women with PID who receive adequate antimicrobial therapy will typically show significant clinical improvement within 72 hours after initiation of therapy. In this setting of acute PID, clinical improvement is demonstrated by resolution of fever, reduced or absent abdominal tenderness, and decreased or absent uterine, adnexal, and cervical motion tenderness.[7] Women managed in the outpatient setting who do not show improvement within 72 hours usually require hospitalization, with a reassessment of the antimicrobial regimen, additional diagnostic tests, and possible surgical intervention.[7] Women diagnosed with chlamydial or gonococcal infections have a high rate of reinfection within 6 months of treatment. Retesting of all women with PID who have been diagnosed with chlamydia or gonorrhea is recommended 3 months after treatment, regardless of whether their sex partners were treated.[7] If retesting at 3 months is not feasible, then retesting should at least occur within 1 year after treatment.[7] There are no specific recommendations for follow-up regarding possible long-term sequelae after treatment for PID or tubo-ovarian abscess.
Management of Sex Partners
All sex partners during the 60 days preceding onset of the woman’s PID symptoms or diagnosis should be examined, tested, and presumptively treated for gonorrhea and chlamydia, regardless of the pathogens identified.[7] If the woman’s last sex partner was more than 60 days before onset of symptoms or diagnosis, then her most recent sex partner should be treated. Expedited partner therapy may be utilized if partner treatment is unlikely to occur. Such evaluation and treatment are imperative because of the risk for reinfection and the strong likelihood of gonococcal or chlamydial infection in the sex partner. Patients (and ideally partners) should be counseled that:
- Male partners of women who have PID caused by C. trachomatis or N. gonorrhoeae are often asymptomatic.
- Sex partners of women with PID should be treated empirically with regimens effective against both C. trachomatis and N. gonorrhoeae, regardless of the apparent etiology of PID or pathogens isolated from the woman with PID.
Counseling and Education
The following summarizes key counseling messages for women diagnosed with PID.
- Resuming Sexual Activity: Women treated for PID should receive instructions to abstain from sexual activity until all the following criteria are met: (1) they have completed treatment for PID, (2) symptoms have resolved, and (3) their sex partners have received appropriate treatment.
- Partner Notification: It is extremely important that women treated for PID understand the importance of partner notification (for all sex partners in the prior 60 days). Partner notification with evaluation and treatment can markedly reduce the spread of STIs in the community, and it also reduces the woman’s likelihood of reinfection and recurrence of PID.
- Follow-Up Testing: It is important that all persons treated for PID have a follow-up visit in approximately 3 months for repeat STI testing.
- STI Prevention: At the time the woman is receiving treatment for PID, it is appropriate to provide counseling messages on how to prevent STIs in the future (e.g., limiting the number of sex partners and consistently using condoms).
- Natural History Following Diagnosis of PID: Women should receive counseling that recurrences of PID may occur. In addition, they should receive counseling that they may experience longer-term complications of PID, including ectopic pregnancy, chronic pelvic pain, and infertility.
Summary Points
- Pelvic inflammatory disease (PID) is a clinical syndrome comprising a spectrum of infectious and inflammatory diseases characterized by ascending spread of organisms from the vagina or cervix to the structures of the upper female genital tract, which may result in endometritis, salpingitis, tubo-ovarian abscess, or perihepatitis.
- Multiple sex partners, age younger than 25 years, and current or prior infection with gonorrhea or chlamydia have consistently been demonstrated to be significant factors associated with enhanced risk of developing PID. The trend of decreasing PID since the year 2009 is likely attributed to an increase in effective screening and treatment of chlamydial and gonococcal infections in adolescents and young women.
- Most cases of PID are polymicrobial and most commonly caused by N. gonorrhoeae or C. trachomatis (or both). Other microbes associated with PID include M. genitalium, T. vaginalis, bacterial vaginosis-associated bacteria, and, less frequently, some gastrointestinal and respiratory organisms.
- Acute sequelae of PID include tubo-ovarian abscess and perihepatitis. Long-term sequelae include ectopic pregnancy, infertility, or chronic pelvic pain.
- No single physical examination finding, image, or laboratory test can reliably make a definitive diagnosis; a combination of clinical criteria and laboratory results should be utilized in making the diagnosis of PID.
- The evaluation for PID should include NAAT testing for chlamydia and gonorrhea, saline microscopy of vaginal fluid, urinalysis, and pregnancy testing. Testing with NAAT for M. genitalium should be considered. All persons diagnosed with PID should also have HIV and syphilis testing.
- Prompt presumptive PID treatment should be initiated for sexually active young women (and other women at risk of STIs) if they are experiencing pelvic or lower abdominal pain, if no cause for the illness other than PID can be identified, or if one or more minimum clinical criteria are met.
- Multiple effective oral, intramuscular, and intravenous treatment regimens are available for the treatment of PID.
- Women with PID who receive appropriate antimicrobial therapy will typically show significant clinical improvement within 72 hours of initiation of therapy. Lack of improvement within 72 hours should prompt reassessment and possible hospitalization for women who are being managed in an outpatient setting.
- All recent sex partners of women with PID should receive evaluation and empiric treatment for chlamydia and gonorrhea, regardless of the pathogens identified in the woman with PID. Repeat testing for chlamydia and gonorrhea should be performed 3 months after PID treatment to screen for reinfection.
Check-On-Learning Questions Display Options
These quick questions are meant to keep you on track and check your understanding. They appear throughout the core concepts and are listed here for you to review.Citations
- 1.Brunham RC, Gottlieb SL, Paavonen J. Pelvic inflammatory disease. N Engl J Med. 2015;372:2039-48.[PubMed Abstract] -
- 2.Hillier SL, Bernstein KT, Aral S. A Review of the Challenges and Complexities in the Diagnosis, Etiology, Epidemiology, and Pathogenesis of Pelvic Inflammatory Disease. J Infect Dis. 2021;224:S23-S28.[PubMed Abstract] -
- 3.Mitchell CM, Anyalechi GE, Cohen CR, Haggerty CL, Manhart LE, Hillier SL. Etiology and Diagnosis of Pelvic Inflammatory Disease: Looking Beyond Gonorrhea and Chlamydia. J Infect Dis. 2021;224:S29-S35.[PubMed Abstract] -
- 4.Soper DE, Wiesenfeld HC. The Continued Challenges in the Diagnosis of Acute Pelvic Inflammatory Disease: Focus on Clinically Mild Disease. J Infect Dis. 2021;224:S75-S79.[PubMed Abstract] -
- 5.Curry A, Williams T, Penny ML. Pelvic Inflammatory Disease: Diagnosis, Management, and Prevention. Am Fam Physician. 2019;100:357-64.[PubMed Abstract] -
- 6.Wiesenfeld HC, Hillier SL, Meyn LA, Amortegui AJ, Sweet RL. Subclinical pelvic inflammatory disease and infertility. Obstet Gynecol. 2012;120:37-43.[PubMed Abstract] -
- 7.Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. Pelvic inflammatory disease (PID). MMWR Recomm Rep. 2021;70(No. RR-4):1-187.
- 8.Kreisel KM, Llata E, Haderxhanaj L, et al. The burden of and trends in pelvic inflammatory disease in the United States, 2006-2016. J Infect Dis. 2021;224:S103-S112.[PubMed Abstract] -
- 9.Kreisel K, Torrone E, Bernstein K, Hong J, Gorwitz R. Prevalence of pelvic inflammatory disease in sexually experienced women of reproductive age - United States, 2013-2014. MMWR Morb Mortal Wkly Rep. 2017;66:80-3.[PubMed Abstract] -
- 10.Bohm MK, Newman L, Satterwhite CL, Tao G, Weinstock HS. Pelvic inflammatory disease among privately insured women, United States, 2001-2005. Sex Transm Dis. 2010;37:131-6.[PubMed Abstract] -
- 11.Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2015. STDs in women and infants. Atlanta: U.S. Department of Health and Human Services; 2016.[PubMed Abstract] -
- 12.Woodhall SC, Torrone L, Fine D, et al. How do changes in the population tested for chlamydia over time affect observed trends in chlamydia positivity? Analysis of routinely collected data from young women tested for chlamydia in family planning clinics in the Pacific Northwest (USA), between 2003 and 2010. Sex Health. 2015;12:512-9.[PubMed Abstract] -
- 13.Anschuetz GL, Asbel L, Spain CV, et al. Association between enhanced screening for Chlamydia trachomatis and Neisseria gonorrhoeae and reductions in sequelae among women. J Adolesc Health. 2012;51:80-5.[PubMed Abstract] -
- 14.Ross JD, Hughes G. Why is the incidence of pelvic inflammatory disease falling? BMJ. 2014;348:g1538.[PubMed Abstract] -
- 15.Davis A, Gaynor A. A comparison of US clinical laboratory Chlamydia and Gonorrhea testing practices before and after the 2014 Centers for Disease Control and Prevention Testing Recommendations. Sex Transm Dis. 2021;48:e73-e76.[PubMed Abstract] -
- 16.Lehto A, Susi A, Prabhakar S, et al. Impact of the COVID-19 Pandemic on Chlamydia, Gonorrhea, and Pelvic Inflammatory Disease Encounters in a United States Uniformed Service Female Beneficiary Population. Mil Med. 2025;190:33-9.[PubMed Abstract] -
- 17.Hay PE, Kerry SR, Normansell R, et al. Which sexually active young female students are most at risk of pelvic inflammatory disease? A prospective study. Sex Transm Infect. 2016;92:63-6.[PubMed Abstract] -
- 18.Jossens MO, Eskenazi B, Schachter J, Sweet RL. Risk factors for pelvic inflammatory disease. A case control study. Sex Transm Dis. 1996;23:239-47.[PubMed Abstract] -
- 19.Leichliter JS, Chandra A, Aral SO. Correlates of self-reported pelvic inflammatory disease treatment in sexually experienced reproductive-aged women in the United States, 1995 and 2006-2010. Sex Transm Dis. 2013;40:413-8.[PubMed Abstract] -
- 20.Eschenbach DA. Acute pelvic inflammatory disease. Urol Clin North Am. 1984;11:65-81.[PubMed Abstract] -
- 21.Ness RB, Randall H, Richter HE, et al. Condom use and the risk of recurrent pelvic inflammatory disease, chronic pelvic pain, or infertility following an episode of pelvic inflammatory disease. Am J Public Health. 2004;94:1327-9.[PubMed Abstract] -
- 22.Scholes D, Stergachis A, Heidrich FE, Andrilla H, Holmes KK, Stamm WE. Prevention of pelvic inflammatory disease by screening for cervical chlamydial infection. N Engl J Med. 1996;334:1362-6.[PubMed Abstract] -
- 23.Oakeshott P, Kerry S, Aghaizu A, et al. Randomised controlled trial of screening for Chlamydia trachomatis to prevent pelvic inflammatory disease: the POPI (prevention of pelvic infection) trial. BMJ. 2010;340:c1642.[PubMed Abstract] -
- 24.Washington AE, Aral SO, Wølner-Hanssen P, Grimes DA, Holmes KK. Assessing risk for pelvic inflammatory disease and its sequelae. JAMA. 1991;266:2581-6.[PubMed Abstract] -
- 25.Ness RB, Kip KE, Hillier SL, et al. A cluster analysis of bacterial vaginosis-associated microflora and pelvic inflammatory disease. Am J Epidemiol. 2005;162:585-90.[PubMed Abstract] -
- 26.Jatlaoui TC, Riley HEM, Curtis KM. The safety of intrauterine devices among young women: a systematic review. Contraception. 2017;95:17-39.[PubMed Abstract] -
- 27.Grimes DA. Intrauterine device and upper-genital-tract infection. Lancet. 2000;356:1013-9.[PubMed Abstract] -
- 28.Carr S, Espey E. Intrauterine devices and pelvic inflammatory disease among adolescents. J Adolesc Health. 2013;52:S22-8.[PubMed Abstract] -
- 29.Nguyen AT, Curtis KM, Tepper NK, et al. U.S. Medical Eligibility Criteria for Contraceptive Use, 2024. MMWR Recomm Rep. 2024;73:1-126.[PubMed Abstract] -
- 30.Turok DK, Eisenberg DL, Teal SB, Keder LM, Creinin MD. A prospective assessment of pelvic infection risk following same-day sexually transmitted infection testing and levonorgestrel intrauterine system placement. Am J Obstet Gynecol. 2016;215:599.e1-599.e6.[PubMed Abstract] -
- 31.Birgisson NE, Zhao Q, Secura GM, Madden T, Peipert JF. Positive Testing for Neisseria gonorrhoeae and Chlamydia trachomatis and the Risk of Pelvic Inflammatory Disease in IUD Users. J Womens Health (Larchmt). 2015;24:354-9.[PubMed Abstract] -
- 32.Sufrin CB, Postlethwaite D, Armstrong MA, Merchant M, Wendt JM, Steinauer JE. Neisseria gonorrhea and Chlamydia trachomatis screening at intrauterine device insertion and pelvic inflammatory disease. Obstet Gynecol. 2012;120:1314-21.[PubMed Abstract] -
- 33.Eschenbach DA, Buchanan TM, Pollock HM, et al. Polymicrobial etiology of acute pelvic inflammatory disease. N Engl J Med. 1975;293:166-71.[PubMed Abstract] -
- 34.Wasserheit JN, Bell TA, Kiviat NB, et al. Microbial causes of proven pelvic inflammatory disease and efficacy of clindamycin and tobramycin. Ann Intern Med. 1986;104:187-93.[PubMed Abstract] -
- 35.Ness RB, Soper DE, Holley RL, et al. Effectiveness of inpatient and outpatient treatment strategies for women with pelvic inflammatory disease: results from the Pelvic Inflammatory Disease Evaluation and Clinical Health (PEACH) Randomized Trial. Am J Obstet Gynecol. 2002;186:929-37.[PubMed Abstract] -
- 36.Pelvic inflammatory disease: guidelines for prevention and management. MMWR Recomm Rep. 1991;40:1-25.[PubMed Abstract] -
- 37.Wiesenfeld HC, Meyn LA, Darville T, Macio IS, Hillier SL. A randomized controlled trial of ceftriaxone and doxycycline, with or without metronidazole, for the treatment of acute pelvic inflammatory disease. Clin Infect Dis. 2021;72:1181-9.[PubMed Abstract] -
- 38.Burnett AM, Anderson CP, Zwank MD. Laboratory-confirmed gonorrhea and/or chlamydia rates in clinically diagnosed pelvic inflammatory disease and cervicitis. Am J Emerg Med. 2012;30:1114-7.[PubMed Abstract] -
- 39.Haggerty CL, Taylor BD. Mycoplasma genitalium: an emerging cause of pelvic inflammatory disease. Infect Dis Obstet Gynecol. 2011;2011:959816.[PubMed Abstract] -
- 40.Ness RB, Hillier SL, Kip KE, et al. Bacterial vaginosis and risk of pelvic inflammatory disease. Obstet Gynecol. 2004;104:761-9.[PubMed Abstract] -
- 41.Taylor BD, Darville T, Haggerty CL. Does bacterial vaginosis cause pelvic inflammatory disease? Sex Transm Dis. 2013;40:117-22.[PubMed Abstract] -
- 42.Htaik K, Vodstrcil LA, Plummer EL, et al. Systematic review and meta-analysis of the association between Mycoplasma genitalium and Pelvic inflammatory disease (PID). Clin Infect Dis. 2024 Jun 7. Online ahead of print.[PubMed Abstract] -
- 43.Taylor BD, Darville T, Ferrell RE, Kammerer CM, Ness RB, Haggerty CL. Variants in toll-like receptor 1 and 4 genes are associated with Chlamydia trachomatis among women with pelvic inflammatory disease. J Infect Dis. 2012;205:603-9.[PubMed Abstract] -
- 44.Verweij SP, Karimi O, Pleijster J, et al. TLR2, TLR4 and TLR9 genotypes and haplotypes in the susceptibility to and clinical course of Chlamydia trachomatis infections in Dutch women. Pathog Dis. 2016;74:ftv107.[PubMed Abstract] -
- 45.Brunham RC, Binns B, Guijon F, et al. Etiology and outcome of acute pelvic inflammatory disease. J Infect Dis. 1988;158:510-7.[PubMed Abstract] -
- 46.Brunham RC. Immunology. A Chlamydia vaccine on the horizon. Science. 2015;348:1322-3.[PubMed Abstract] -
- 47.Wiesenfeld HC, Hillier SL, Krohn MA, et al. Lower genital tract infection and endometritis: insight into subclinical pelvic inflammatory disease. Obstet Gynecol. 2002;100:456-63.[PubMed Abstract] -
- 48.Wiesenfeld HC, Sweet RL, Ness RB, Krohn MA, Amortegui AJ, Hillier SL. Comparison of acute and subclinical pelvic inflammatory disease. Sex Transm Dis. 2005;32:400-5.[PubMed Abstract] -
- 49.Eschenbach DA, Wölner-Hanssen P, Hawes SE, Pavletic A, Paavonen J, Holmes KK. Acute pelvic inflammatory disease: associations of clinical and laboratory findings with laparoscopic findings. Obstet Gynecol. 1997;89:184-92.[PubMed Abstract] -
- 50.Rosen M, Breitkopf D, Waud K. Tubo-ovarian abscess management options for women who desire fertility. Obstet Gynecol Surv. 2009;64:681-9.[PubMed Abstract] -
- 51.Peter NG, Clark LR, Jaeger JR. Fitz-Hugh-Curtis syndrome: a diagnosis to consider in women with right upper quadrant pain. Cleve Clin J Med. 2004;71:233-9.[PubMed Abstract] -
- 52.Lareau SM, Beigi RH. Pelvic inflammatory disease and tubo-ovarian abscess. Infect Dis Clin North Am. 2008;22:693-708.[PubMed Abstract] -
- 53.Hmaidan S , Mutter O, Muldoon, G. Moawad, A. Yunker A. Laparoscopic and robotic management of perihepatic adhesions. Am J Obstet Gynecol. 2022;226(3):Supplement S1358-S1359.[AJOG] -
- 54.Landers DV, Sweet RL. Current trends in the diagnosis and treatment of tuboovarian abscess. Am J Obstet Gynecol. 1985;151:1098-110.[PubMed Abstract] -
- 55.Chappell CA, Wiesenfeld HC. Pathogenesis, diagnosis, and management of severe pelvic inflammatory disease and tuboovarian abscess. Clin Obstet Gynecol. 2012;55:893-903.[PubMed Abstract] -
- 56.Chayachinda C, Rekhawasin T. Reproductive outcomes of patients being hospitalised with pelvic inflammatory disease. J Obstet Gynaecol. 2016;:1-5.[PubMed Abstract] -
- 57.Heinonen PK, Leinonen M. Fecundity and morbidity following acute pelvic inflammatory disease treated with doxycycline and metronidazole. Arch Gynecol Obstet. 2003;268:284-8.[PubMed Abstract] -
- 58.Hillis SD, Joesoef R, Marchbanks PA, Wasserheit JN, Cates W Jr, Westrom L. Delayed care of pelvic inflammatory disease as a risk factor for impaired fertility. Am J Obstet Gynecol. 1993;168:1503-9.[PubMed Abstract] -
- 59.Weström L, Joesoef R, Reynolds G, Hagdu A, Thompson SE. Pelvic inflammatory disease and fertility. A cohort study of 1,844 women with laparoscopically verified disease and 657 control women with normal laparoscopic results. Sex Transm Dis. 1992;19:185-92.[PubMed Abstract] -
- 60.Recent advances in medically assisted conception. Report of a WHO Scientific Group. World Health Organ Tech Rep Ser. 1992;820:1-111.[PubMed Abstract] -
- 61.French CE, Hughes G, Nicholson A, et al. Estimation of the rate of pelvic inflammatory disease diagnoses: trends in England, 2000-2008. Sex Transm Dis. 2011;38:158-62.[PubMed Abstract] -
- 62.Dun EC, Nezhat CH. Tubal factor infertility: diagnosis and management in the era of assisted reproductive technology. Obstet Gynecol Clin North Am. 2012;39:551-66.[PubMed Abstract] -
- 63.Kahn JG, Walker CK, Washington AE, Landers DV, Sweet RL. Diagnosing pelvic inflammatory disease. A comprehensive analysis and considerations for developing a new model. JAMA. 1991;266:2594-604.[PubMed Abstract] -
- 64.Gaitán H, Angel E, Diaz R, Parada A, Sanchez L, Vargas C. Accuracy of five different diagnostic techniques in mild-to-moderate pelvic inflammatory disease. Infect Dis Obstet Gynecol. 2002;10:171-80.[PubMed Abstract] -
- 65.Sellors J, Mahony J, Goldsmith C, et al. The accuracy of clinical findings and laparoscopy in pelvic inflammatory disease. Am J Obstet Gynecol. 1991;164:113-20.[PubMed Abstract] -
- 66.Jacobson L, Weström L. Objectivized diagnosis of acute pelvic inflammatory disease. Diagnostic and prognostic value of routine laparoscopy. Am J Obstet Gynecol. 1969;105:1088-98.[PubMed Abstract] -
- 67.Livengood CH 3rd, Hill GB, Addison WA. Pelvic inflammatory disease: findings during inpatient treatment of clinically severe, laparoscopy-documented disease. Am J Obstet Gynecol. 1992;166:519-24.[PubMed Abstract] -
- 68.Peipert JF, Boardman LA, Sung CJ. Performance of clinical and laparoscopic criteria for the diagnosis of upper genital tract infection. Infect Dis Obstet Gynecol. 1997;5:291-6.[PubMed Abstract] -
- 69.Haggerty CL, Ness RB. Newest approaches to treatment of pelvic inflammatory disease: a review of recent randomized clinical trials. Clin Infect Dis. 2007;44:953-60.[PubMed Abstract] -
- 70.McGregor JA, Crombleholme WR, Newton E, Sweet RL, Tuomala R, Gibbs RS. Randomized comparison of ampicillin-sulbactam to cefoxitin and doxycycline or clindamycin and gentamicin in the treatment of pelvic inflammatory disease or endometritis. Obstet Gynecol. 1994;83:998-1004.[PubMed Abstract] -
- 71.Savaris RF, Teixeira LM, Torres TG, Edelweiss MI, Moncada J, Schachter J. Comparing ceftriaxone plus azithromycin or doxycycline for pelvic inflammatory disease: a randomized controlled trial. Obstet Gynecol. 2007;110:53-60.[PubMed Abstract] -
- 72.Sweet RL, Schachter J, Landers DV, Ohm-Smith M, Robbie MO. Treatment of hospitalized patients with acute pelvic inflammatory disease: comparison of cefotetan plus doxycycline and cefoxitin plus doxycycline. Am J Obstet Gynecol. 1988;158:736-41.[PubMed Abstract] -
- 73.Sweet RL. Treatment of acute pelvic inflammatory disease. Infect Dis Obstet Gynecol. 2011;2011:561909.[PubMed Abstract] -
- 74.Walters MD, Gibbs RS. A randomized comparison of gentamicin-clindamycin and cefoxitin-doxycycline in the treatment of acute pelvic inflammatory disease. Obstet Gynecol. 1990;75:867-72.[PubMed Abstract] -
- 75.Hemsell DL, Little BB, Faro S, et al. Comparison of three regimens recommended by the Centers for Disease Control and Prevention for the treatment of women hospitalized with acute pelvic inflammatory disease. Clin Infect Dis. 1994;19:720-7.[PubMed Abstract] -
- 76.Walker CK, Wiesenfeld HC. Antibiotic therapy for acute pelvic inflammatory disease: the 2006 Centers for Disease Control and Prevention sexually transmitted diseases treatment guidelines. Clin Infect Dis. 2007;44 Suppl 3:S111-22.[PubMed Abstract] -
- 77.Bevan CD, Ridgway GL, Rothermel CD. Efficacy and safety of azithromycin as monotherapy or combined with metronidazole compared with two standard multidrug regimens for the treatment of acute pelvic inflammatory disease. J Int Med Res. 2003;31:45-54.[PubMed Abstract] -
- 78.Judlin P, Liao Q, Liu Z, Reimnitz P, Hampel B, Arvis P. Efficacy and safety of moxifloxacin in uncomplicated pelvic inflammatory disease: the MONALISA study. BJOG. 2010;117:1475-84.[PubMed Abstract] -
- 79.Ross JD, Cronjé HS, Paszkowski T, et al. Moxifloxacin versus ofloxacin plus metronidazole in uncomplicated pelvic inflammatory disease: results of a multicentre, double blind, randomised trial. Sex Transm Infect. 2006;82:446-51.[PubMed Abstract] -
- 80.Heystek M, Ross JD. A randomized double-blind comparison of moxifloxacin and doxycycline/metronidazole/ciprofloxacin in the treatment of acute, uncomplicated pelvic inflammatory disease. Int J STD AIDS. 2009;20:690-5.[PubMed Abstract] -
- 81.Risser WL, Risser JM, Risser AL. Current perspectives in the USA on the diagnosis and treatment of pelvic inflammatory disease in adolescents. Adolesc Health Med Ther. 2017;8:87-94.[PubMed Abstract] -
- 82.Trent M, Perin J, Gaydos CA, et al. Efficacy of a Technology-Enhanced Community Health Nursing Intervention vs Standard of Care for Female Adolescents and Young Adults With Pelvic Inflammatory Disease: A Randomized Clinical Trial. JAMA Netw Open. 2019;2:e198652.[PubMed Abstract] -
- 83.Trent M, Perin J, Rowell J, et al. Using Innovation to Address Adolescent and Young Adult Health Disparities in Pelvic Inflammatory Disease: Design of the Technology Enhanced Community Health Precision Nursing (TECH-PN) Trial. J Infect Dis. 2021;224:S145-S151.[PubMed Abstract] -
- 84.Ballreich J, Frick KD, Huettner S, et al. Cost-effectiveness Analysis of the Technology-Enhanced Community Health Nursing Program for Adolescent Girls and Young Adult Women with Pelvic Inflammatory Disease. Sex Transm Dis. 2025 Feb 20. Online Adhead of Print.[PubMed Abstract] -
- 85.Korn AP, Landers DV, Green JR, Sweet RL. Pelvic inflammatory disease in human immunodeficiency virus-infected women. Obstet Gynecol. 1993;82:765-8.[PubMed Abstract] -
- 86.Sweet RL, Landers DV. Pelvic inflammatory disease in HIV-positive women. Lancet. 1997;349:1265-6.[PubMed Abstract] -
- 87.Barbosa C, Macasaet M, Brockmann S, Sierra MF, Xia Z, Duerr A. Pelvic inflammatory disease and human immunodeficiency virus infection. Obstet Gynecol. 1997;89:65-70.[PubMed Abstract] -
- 88.Mugo NR, Kiehlbauch JA, Nguti R, et al. Effect of human immunodeficiency virus-1 infection on treatment outcome of acute salpingitis. Obstet Gynecol. 2006;107:807-12.[PubMed Abstract] -
- 89.Irwin KL, Moorman AC, O'Sullivan MJ, et al. Influence of human immunodeficiency virus infection on pelvic inflammatory disease. Obstet Gynecol. 2000;95:525-34.[PubMed Abstract] -
- 90.Bukusi EA, Cohen CR, Stevens CE, et al. Effects of human immunodeficiency virus 1 infection on microbial origins of pelvic inflammatory disease and on efficacy of ambulatory oral therapy. Am J Obstet Gynecol. 1999;181:1374-81.[PubMed Abstract] -
- 91.Reed SD, Landers DV, Sweet RL. Antibiotic treatment of tuboovarian abscess: comparison of broad-spectrum beta-lactam agents versus clindamycin-containing regimens. Am J Obstet Gynecol. 1991;164:1556-61.[PubMed Abstract] -
- 92.Wiesenfeld HC, Sweet RL. Progress in the management of tuboovarian abscesses. Clin Obstet Gynecol. 1993;36:433-44.[PubMed Abstract] -
- 93.Tepper NK, Steenland MW, Gaffield ME, Marchbanks PA, Curtis KM. Retention of intrauterine devices in women who acquire pelvic inflammatory disease: a systematic review. Contraception. 2013;87:655-60.[PubMed Abstract] -
Additional References
- Bender N, Herrmann B, Andersen B, et al. Chlamydia infection, pelvic inflammatory disease, ectopic pregnancy and infertility: cross-national study. Sex Transm Infect. 2011;87:601-8.[PubMed Abstract] -
- Bjartling C, Osser S, Persson K. Mycoplasma genitalium in cervicitis and pelvic inflammatory disease among women at a gynecologic outpatient service. Am J Obstet Gynecol. 2012;206:476.e1-8.[PubMed Abstract] -
- Boothby M, Page J, Pryor R, Ross JD. A comparison of treatment outcomes for moxifloxacin versus ofloxacin/metronidazole for first-line treatment of uncomplicated non-gonococcal pelvic inflammatory disease. Int J STD AIDS. 2010;21:195-7.[PubMed Abstract] -
- Han T, Nolan SM, Regard M. Mycoplasma genitalium as a Cause of Pelvic Inflammatory Disease. J Pediatr Adolesc Gynecol. 2020;33:739-41.[PubMed Abstract] -
- He D, Wang T, Ren W. Global burden of pelvic inflammatory disease and ectopic pregnancy from 1990 to 2019. BMC Public Health. 2023;23:1894.[PubMed Abstract] -
- Mikamo H, Iwasaku K, Yamagishi Y, Matsumizu M, Nagashima M. Efficacy and safety of intravenous azithromycin followed by oral azithromycin for the treatment of acute pelvic inflammatory disease and perihepatitis in Japanese women. J Infect Chemother. 2014;20:429-35.[PubMed Abstract] -
- Rekart ML, Gilbert M, Meza R, et al. Chlamydia public health programs and the epidemiology of pelvic inflammatory disease and ectopic pregnancy. J Infect Dis. 2013;207:30-8.[PubMed Abstract] -
- Romosan G, Valentin L. The sensitivity and specificity of transvaginal ultrasound with regard to acute pelvic inflammatory disease: a review of the literature. Arch Gynecol Obstet. 2014;289:705-14.[PubMed Abstract] -
- Scutiero G, Taliento C, Vizzielli G, et al. Evolving trends in the management of pelvic inflammatory disease (PID) during SARS-CoV-2 pandemic: A multicenter retrospective cohort study. J Obstet Gynaecol Res. 2024;50:1362-7.[PubMed Abstract] -
- Short VL, Totten PA, Ness RB, Astete SG, Kelsey SF, Haggerty CL. Clinical presentation of Mycoplasma genitalium infection versus Neisseria gonorrhoeae infection among women with pelvic inflammatory disease. Clin Infect Dis. 2009;48:41-7.[PubMed Abstract] -
- Viberga I, Odlind V, Lazdane G, Kroica J, Berglund L, Olofsson S. Microbiology profile in women with pelvic inflammatory disease in relation to IUD use. Infect Dis Obstet Gynecol. 2005;13:183-90.[PubMed Abstract] -
- Woods JL, Scurlock AM, Hensel DJ. Pelvic inflammatory disease in the adolescent: understanding diagnosis and treatment as a health care provider. Pediatr Emerg Care. 2013;29:720-5.[PubMed Abstract] -
Figures
 Figure 1. Clinical Classification of Pelvic Inflammatory DiseaseSource: Brunham RC, Gottlieb SL, Paavonen J. Pelvic inflammatory disease. N Engl J Med. 2015;372:2039-48.
Figure 1. Clinical Classification of Pelvic Inflammatory DiseaseSource: Brunham RC, Gottlieb SL, Paavonen J. Pelvic inflammatory disease. N Engl J Med. 2015;372:2039-48. Figure 2. Prevalence of Self-Reported Lifetime PID and Age of Sexual DebutSource: Kreisel K, Torrone E, Bernstein K, Hong J, Gorwitz R. Prevalence of Pelvic Inflammatory Disease in Sexually Experienced Women of Reproductive Age - United States, 2013-2014. MMWR Morb Mortal Wkly Rep. 2017;66:80-3.
Figure 2. Prevalence of Self-Reported Lifetime PID and Age of Sexual DebutSource: Kreisel K, Torrone E, Bernstein K, Hong J, Gorwitz R. Prevalence of Pelvic Inflammatory Disease in Sexually Experienced Women of Reproductive Age - United States, 2013-2014. MMWR Morb Mortal Wkly Rep. 2017;66:80-3. Figure 3. Prevalence of Self-Reported Lifetime PID and Number of Sexual PartnersSource: Kreisel K, Torrone E, Bernstein K, Hong J, Gorwitz R. Prevalence of Pelvic Inflammatory Disease in Sexually Experienced Women of Reproductive Age - United States, 2013-2014. MMWR Morb Mortal Wkly Rep. 2017;66:80-3.
Figure 3. Prevalence of Self-Reported Lifetime PID and Number of Sexual PartnersSource: Kreisel K, Torrone E, Bernstein K, Hong J, Gorwitz R. Prevalence of Pelvic Inflammatory Disease in Sexually Experienced Women of Reproductive Age - United States, 2013-2014. MMWR Morb Mortal Wkly Rep. 2017;66:80-3. Figure 4. Ascending Bacterial Infection with Potential to Cause Pelvic Inflammatory DiseaseIllustration by Cognition Studio, Inc. and David H. Spach, MD
Figure 4. Ascending Bacterial Infection with Potential to Cause Pelvic Inflammatory DiseaseIllustration by Cognition Studio, Inc. and David H. Spach, MD Figure 5. Pathologic Changes in the Epithelial Surface of the Fallopian Tube after PIDScanning electron micrographs show normal human fallopian tube epithelia (Panel A) and the epithelial surface after PID (Panel B). A selective loss of ciliated epithelial cells occurs after PID, which interferes with intratubal ovum transport. Original images Dorothy L. Patton, University of Washington, Seattle.Source: Brunham RC, Gottlieb SL, Paavonen J. Pelvic inflammatory disease. N Engl J Med. 2015;372:2039-48. Reproduced with permission. Massachusetts Medical Society. ©2015.
Figure 5. Pathologic Changes in the Epithelial Surface of the Fallopian Tube after PIDScanning electron micrographs show normal human fallopian tube epithelia (Panel A) and the epithelial surface after PID (Panel B). A selective loss of ciliated epithelial cells occurs after PID, which interferes with intratubal ovum transport. Original images Dorothy L. Patton, University of Washington, Seattle.Source: Brunham RC, Gottlieb SL, Paavonen J. Pelvic inflammatory disease. N Engl J Med. 2015;372:2039-48. Reproduced with permission. Massachusetts Medical Society. ©2015. Figure 6 (Image Series). Acute SalpingitisWith acute PID, women may develop salpingitis and marked fallopian tube swelling. This may be accompanied by fallopian adhesions, tube obstruction, and the development of a tubo-ovarian abscess.Illustration by Cognition Studio, Inc. and David H. Spach, MD
Figure 6 (Image Series). Acute SalpingitisWith acute PID, women may develop salpingitis and marked fallopian tube swelling. This may be accompanied by fallopian adhesions, tube obstruction, and the development of a tubo-ovarian abscess.Illustration by Cognition Studio, Inc. and David H. Spach, MD Figure 6B. Acute Salpingitis with Tubal Adhesions and Tubal NarrowingWith acute PID, women may develop tubal adhesions, tubal narrowing, and tubal occlusion.Illustration by Cognition Studio, Inc. and David H. Spach, MD
Figure 6B. Acute Salpingitis with Tubal Adhesions and Tubal NarrowingWith acute PID, women may develop tubal adhesions, tubal narrowing, and tubal occlusion.Illustration by Cognition Studio, Inc. and David H. Spach, MD Figure 7 (Image Series). Fitz Hugh Curtis SyndromeIllustration by Cognition Studio, Inc. and David H. Spach, MD
Figure 7 (Image Series). Fitz Hugh Curtis SyndromeIllustration by Cognition Studio, Inc. and David H. Spach, MD Figure 7B. Close up View of Adhesions Between Liver and Parietal PeritoneumIllustration by Cognition Studio, Inc. and David H. Spach, MD
Figure 7B. Close up View of Adhesions Between Liver and Parietal PeritoneumIllustration by Cognition Studio, Inc. and David H. Spach, MD Figure 7C. Violin String AdhesionsIllustration by Cognition Studio, Inc. and David H. Spach, MD
Figure 7C. Violin String AdhesionsIllustration by Cognition Studio, Inc. and David H. Spach, MD Figure 8. Tubo-Ovarian AbscessIllustration by Cognition Studio, Inc. and David H. Spach, MD
Figure 8. Tubo-Ovarian AbscessIllustration by Cognition Studio, Inc. and David H. Spach, MD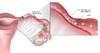 Figure 9 (Image Series). Post-Salpingitis Tubal Scarring and ObstructionFollowing an episode of PID and acute salpingitis, adhesions and tubal narrowing can cause infertility due to the blockage of ovum passage through the fallopian tube.Illustration by Cognition Studio, Inc. and David H. Spach, MD
Figure 9 (Image Series). Post-Salpingitis Tubal Scarring and ObstructionFollowing an episode of PID and acute salpingitis, adhesions and tubal narrowing can cause infertility due to the blockage of ovum passage through the fallopian tube.Illustration by Cognition Studio, Inc. and David H. Spach, MD Figure 9B. Obstructed Fallopian TubeClose up view of tubal narrowing, adhesions, and obstruction of ovum passage through the fallopian tube.Illustration by Cognition Studio, Inc. and David H. Spach, MD
Figure 9B. Obstructed Fallopian TubeClose up view of tubal narrowing, adhesions, and obstruction of ovum passage through the fallopian tube.Illustration by Cognition Studio, Inc. and David H. Spach, MDTables
Table 1. Clinical Classification of Pelvic Inflammatory Disease and Likely Microbial Causes
Clinical Syndrome Causes © 2016 Massachusetts Medical Society. Reproduced with permission. Acute pelvic inflammatory disease (≤30 days' duration)
Cervical pathogens (Neisseria gonorrhoeae, Chlamydia trachomatis, and Mycoplasma genitalium)
Bacterial vaginosis pathogens (Peptostreptococcus species, Bacteroides species, Atopobium species, Leptotrichia species, M. hominis, Ureaplasma urealyticum, and Clostridia species)
Respiratory pathogens (Haemophilus influenzae, Streptococcus pneumoniae, group A streptococci, and Staphylococcus aureus)
Enteric pathogens (Escherichia coli, Bacteroides fragilis, group B streptococci, and Campylobacter species)
Subclinical pelvic inflammatory disease C. trachomatis and N. gonorrhoeae Chronic pelvic inflammatory disease (>30 days' duration) Mycobacterium tuberculosis and Actinomyces species Source:- Brunham RC, Gottlieb SL, Paavonen J. Pelvic inflammatory disease. N Engl J Med. 2015;372:2039-48. [PubMed Abstract]
Table 2. 2021 STI Treatment Guidelines: Pelvic Inflammatory Disease (PID)Parenteral RegimensClinical experience should guide decisions regarding transition to oral therapy, which usually can be initiated within 24–48 hours of clinical improvement. The oral regimen should be based on the initial parenteral regimen, as outlined below. In women with tubo-ovarian abscesses, at least 24 hours of inpatient observation is recommended.Recommended RegimensCeftriaxone1 g IV every 24 hoursCeftriaxone
Tradename:RocephinDoxycycline100 mg orally or IV every 12 hours*+Doxycycline
Tradename:Doryx, VibramycinMetronidazole500 mg orally or IV every 12 hours^+Metronidazole
Tradename:Flagyl*Because of the pain associated with IV infusion, doxycycline should be administered orally when possible. Oral and IV administration of doxycycline provide similar bioavailability.^Oral metronidazole is well absorbed and can be considered instead of IV for women without severe illness or tubo-ovarian abscess when possible.Note: Within 24 to 48 hours of clinical improvement, therapy can be transitioned to oral therapy with doxycycline 100 mg twice daily plus metronidazole 500 mg twice daily to complete 14 days of antimicrobial therapy.Recommended RegimensCefotetan2 g IV every 12 hoursCefotetan
Tradename:CefotanDoxycycline100 mg orally or IV every 12 hours*+Doxycycline
Tradename:Doryx, Vibramycin*Because of the pain associated with intravenous infusion, doxycycline should be administered orally when possible. Oral and IV administration of doxycycline provide similar bioavailability.Note: Within 24 to 48 hours of clinical improvement, therapy can be transitioned to oral therapy with doxycycline 100 mg twice daily plus metronidazole 500 mg twice daily to complete 14 days of antimicrobial therapy.Recommended RegimensCefoxitin2 g IV every 6 hoursCefoxitin
Tradename:MefoxinDoxycycline100 mg orally or IV every 12 hours+Doxycycline
Tradename:Doryx, VibramycinBecause of the pain associated with intravenous infusion, doxycycline should be administered orally when possible. Oral and IV administration of doxycycline provide similar bioavailability.Note: Within 24 to 48 hours of clinical improvement, therapy can be transitioned to oral therapy with doxycycline 100 mg twice daily plus metronidazole 500 mg twice daily to complete 14 days of antimicrobial therapy.Alternative RegimensAmpicillin-Sulbactam3 g IV every 6 hoursAmpicillin-Sulbactam
Tradename:UnasynDoxycycline100 mg orally or IV every 12 hours*+Doxycycline
Tradename:Doryx, Vibramycin*Because of the pain associated with intravenous infusion, doxycycline should be administered orally when possible. Oral and IV administration of doxycycline provide similar bioavailability.Note: Within 24 to 48 hours of clinical improvement, therapy can be transitioned to oral therapy with doxycycline 100 mg twice daily plus metronidazole 500 mg twice daily to complete 14 days of antimicrobial therapy.Alternative RegimensClindamycin900 mg IV every 8 hoursClindamycin
Tradename:CleocinGentamicinloading dose IV or IM (2 mg/kg body weight), followed by a maintenance dose (1.5 mg/kg body weight) every 8 hours; single daily dosing (3–5 mg/kg body weight) can be substituted+Gentamicin
Tradename:GaramycinNote: Within 24 to 48 hours of clinical improvement, therapy can be transitioned to oral therapy with clindamycin 450 mg four times a day or doxycycline 100 mg twice daily to complete 14 days of antimicrobial therapy.Note: if tubo-ovarian abscess is present, the regimen used when transitioning to oral therapy should consist of doxycycline plus either clindamycin 450 mg four times a day or metronidazole 500 mg twice daily to complete 14 days of antimicrobial therapy.Source: Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. Pelvic inflammatory disease (PID). MMWR Recomm Rep. 2021;70(No. RR-4):1-187. [2021 STI Treatment Guidelines]Table 3. 2021 STI Treatment Guidelines: Pelvic Inflammatory Disease (PID)Intramuscular or Oral RegimensFor women with mild-to-moderate acute PID, intramuscular (IM) or oral therapy can be considered because the clinical outcomes among women treated with these regimens are similar to those treated with IV therapy. Women who do not respond to IM/oral therapy within 72 hours should be reevaluated to confirm the diagnosis and should be administered intravenous therapy.Recommended RegimensCeftriaxone500 mg IM in a single dose*Ceftriaxone
Tradename:RocephinDoxycycline100 mg orally twice a day for 14 days+Doxycycline
Tradename:Doryx, VibramycinMetronidazole500 mg orally twice a day for 14 days+Metronidazole
Tradename:Flagyl*For persons weighing ≥150 kg, 1 gm of ceftriaxone should be administered.Recommended RegimensCefoxitin2 g IM in a single doseCefoxitin
Tradename:MefoxinProbenecid1 g orally in a single dose (given concurrently with cefoxitin)+Probenecid
Tradename:Doxycycline100 mg orally twice a day for 14 days+Doxycycline
Tradename:Doryx, VibramycinMetronidazole500 mg orally twice a day for 14 days+Metronidazole
Tradename:FlagylThe cefoxitin and probenecid should be administered concurrently.Recommended RegimensOther parenteral third-generation cephalosporin (e.g., ceftizoxime or cefotaxime)Other parenteral third-generation cephalosporin (e.g., ceftizoxime or cefotaxime)
Tradename:Doxycycline100 mg orally twice a day for 14 days+Doxycycline
Tradename:Doryx, VibramycinMetronidazole500 mg orally twice a day for 14 days+Metronidazole
Tradename:FlagylAlternative RegimensLevofloxacin500 mg orally once daily for 14 daysLevofloxacin
Tradename:LevaquinMetronidazole500 mg orally twice a day for 14 days+Metronidazole
Tradename:FlagylNote: This alternative regimen is only for consideration if the patient has cephalosporin allergy, the community prevalence and individual risk for gonorrhea are low, and follow-up is likely.Alternative RegimensMoxifloxacin400 mg orally once daily for 14 daysMoxifloxacin
Tradename:AveloxNote: This alternative regimen is only for consideration if the patient has cephalosporin allergy, the community prevalence and individual risk for gonorrhea are low, and follow-up is likely. Moxifloxacin is the preferred quinolone antimicrobial for M. genitalium infections; however, the importance of providing coverage for M. genitalium is unknown.Alternative RegimensAzithromycin500 mg IV daily for 1–2 doses, followed by 250 mg orally daily for a total of 7 daysAzithromycin
Tradename:ZithromaxMetronidazole500 mg orally three times a day for 12-14 days±Metronidazole
Tradename:FlagylNote: This alternative regimen is only for consideration if the patient has cephalosporin allergy, the community prevalence and individual risk for gonorrhea are low, and follow-up is likely.Source: Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. Pelvic inflammatory disease (PID). MMWR Recomm Rep. 2021;70(No. RR-4):1-187. [2021 STI Treatment Guidelines]Share by e-mail
Check
-On-
Learning
QuestionsThe Check-on-Learning Questions are short and topic related. They are meant to help you stay on track throughout each lesson and check your understanding of key concepts.You must be signed in to customize your interaction with these questions.
Since you've received 80% or better on this quiz, you may claim continuing education credit.
You seem to have a popup blocker enabled. If you want to skip this dialog please Always allow popup windows for the online course.
Current Version: nstdc-monorepo-bcea158b-2026-01-22-172200Become a New UserAccount Registration Benefits:
- Track your progress on the lessons
- Earn free CNE/CME/CE
- Earn Certificates of Completion
- Access to other free IDEA curricula
Create a free account to get started
- 0%Lesson 2

















